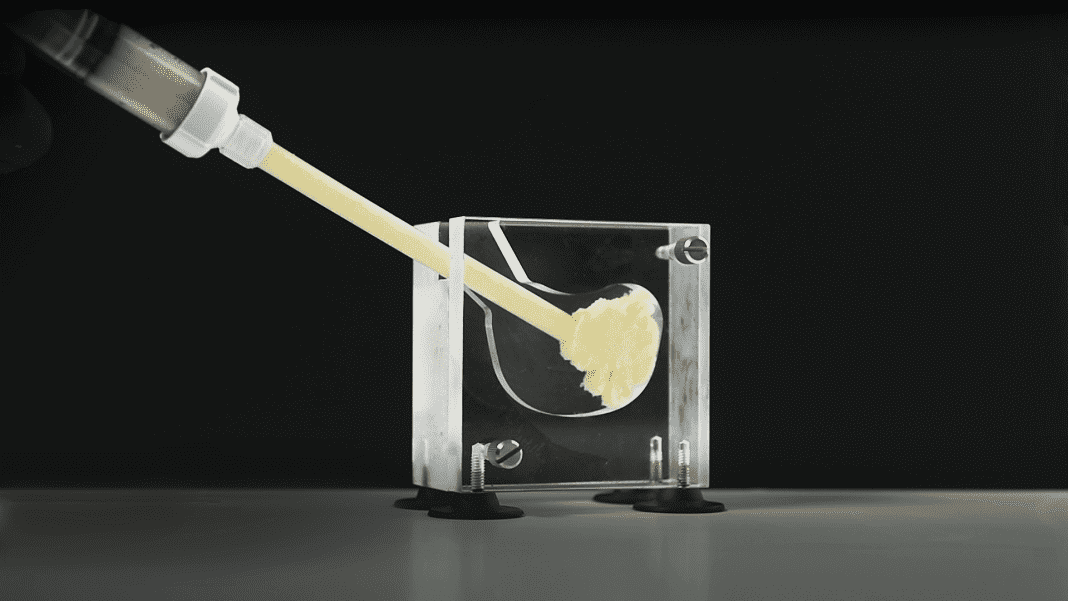
OSTEOAMP SELECT Flowable, a flowable allograft bone graft substitute solution developed for a variety of patient procedures including lumbar spine fusion, cervical spine fusion, and foot & ankle fusion has been launched by Bioventus.
Introduced in a limited release in select US markets beginning in March 2021, OSTEOAMP SELECT Flowable is 100 percent allograft with no synthetic carrier added, yet still based on the unique OSTEOAMP process designed to retain a wide array of essential growth factors. * Its versatile handling is designed to satisfy the need for a flowable allograft product with cohesive properties, making it an attractive option for minimally invasive surgery (MIS), expandable cages and 3D-printed cages.
“Spine, trauma and foot & ankle surgeons are looking for allograft options that handle well for a variety of procedures,” said Dr. Larry Boyd, Vice President, Product Development, Bioventus. “Developed by our team in collaboration with our tissue bank partner, OSTEOAMP SELECT Flowable comes ready-to-use and is designed to be delivered in a range of methods and to provide excellent retention characteristics at the grafting site. It is terminally sterilized and processed using advanced procedures designed to comply with the highest standards for tissue banking, including comprehensive donor screening and extensive microbiological testing.”
“OSTEOAMP SELECT Flowable has been an ideal product for a variety of my minimally invasive interbody fusions, particularly with expandable cage technology, where I need a graft that handles efficiently to navigate tight spaces,” said Dr. Paul Kim, Carolina Neurosurgery & Spine Associates. “I have used various formats of OSTEOAMP for years and have seen firsthand the successful patient outcomes it can help provide.”
“A differentiated allograft product with the handling characteristics like OSTEOAMP SELECT Flowable is an asset for a spine surgeon who uses 3D printed cages where grafting can be challenging,” said Dr. Safdar Khan, Ohio State University. “I was impressed to see how the flowable product filled a cage with a tight, porous structure so well and stayed in place during implantation.”
OSTEOAMP SELECT Flowable comes in three sizes from 2.5 to 10 cc and is available nationwide.
*In vitro performance may not be predictive of performance in humans.