Innovation is one of the driving forces behind medical device manufacturing. As we learn more about medical conditions and their effect on the body, products designed to support patients living with these conditions naturally get better. As a result, design, development, and production processes are iterative.
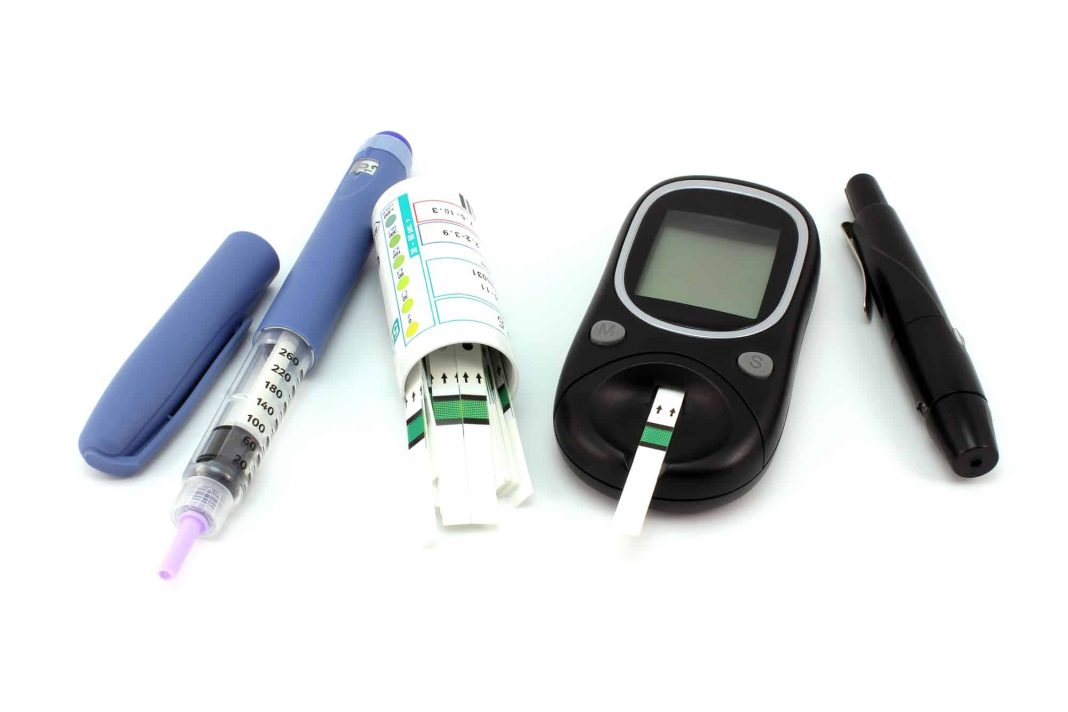
Take something like the common at-home blood glucose test for diabetics. Urine test strips were the first mode of monitoring blood glucose, introduced in the 1940s. The first at-home blood glucose monitor didn’t hit the market until 1970 and for decades after, the finger prick blood test was part of everyday life for diabetics across the world. Today, the world of personal glucose monitoring is vastly different. Reagent strip systems are still commonplace, but there’s a strong movement toward prick-less technologies, such as noninvasive glucose meters.
Even within these macro innovations, the technology powering medical devices is ever-changing. Test strips continued to get better before they were left behind for glucose meters—which today are becoming more accurate as sensor technologies evolve. It begs the question: is it possible to perfect a medical device?
The short answer, is no. And that’s a good thing.
Innovations in healthcare force iteration from medical device manufacturers. The more we learn about an illness or condition, the better we can treat or support it. And, as new technologies and materials become available, it’s almost Hippocratic to ensure they’re leveraged into a better standard of patient care.
But this demand for iterative production doesn’t come without challenge. Medical device manufacturers face many obstacles in improving their products—especially at a pace that marks demand for them. Some of the biggest challenges include:
- Every innovation comes with a dollar value attached. Many times, this cost is unknown until the endeavor is complete. While it’s possible to anticipate new material costs or production fees, there are variables that drive up the cost of innovation that aren’t easy to account for. For example, how much will trialing this updated device cost?
- Insurance companies are notoriously picky when it comes to medical devices. Many exclude devices from coverage if they’re not FDA approved yet. Others may cover v1 of a device, but not v2, claiming unproven features or design. Jumping through regulatory hoops with each new version not only makes iterating difficult, it also actively dissuades device manufacturers from innovating.
- Innovation generally means improvement. Often, it’s in the best interest of a medical device manufacturer to push a new iteration by recalling an old one—whether as a swap or at a discounted rate. The reason? Avoiding lawsuits and delivering an enhanced experience for patients. Facilitating a recall is difficult and costly in the short term, even if it saves money and promotes success long-term.
- More than 80% of medical device companies in the United States are startups or small businesses, employing fewer than 50 people. While this makes them agile, it also encumbers them in terms of manpower and funding. Churning out iterations to meet patient demand and capitalize on new information or materials is often slow-going. It requires decisive leadership with a forward-looking mindset for demand.
- Many medical devices are quickly becoming too complex to iterate without substantial reinvestment or broader development. This is chiefly due to microelectronics, telecommunications, instrumentation and biotechnology considerations. As a result, iterations don’t always pace available capabilities.
These challenges only begin to scrape the surface for medical device manufacturers. Not every product has as much widespread demand or well-funded research behind it as something like a blood glucose monitor. Stepping outside of personal medical devices, electro-medical equipment, in-vitro diagnostics products, irradiation apparatuses and similarly sophisticated products pose much wider and more complex challenges for producers.
Ultimately, these aren’t new challenges for seasoned medical device manufacturers. Moreover, most startups have an acute understanding of these obstacles as they enter the market. The solution? Forward-thinking leadership and a willingness to plan for cyclical iteration. Developing devices for the future means looking in that direction and keenly observing the peripheral factors that may affect the next iteration of a flagship product.
It’s not easy to keep the pace of innovation consistent with demand, much less get out in front of it. Medical device manufacturers already understand this. That means finding solutions in spite of the known obstacles and striving to iterate devices around them. Good leadership is the key, and the guiding, governing factor as medical device makers strive to succeed in an unforgiving, albeit ess