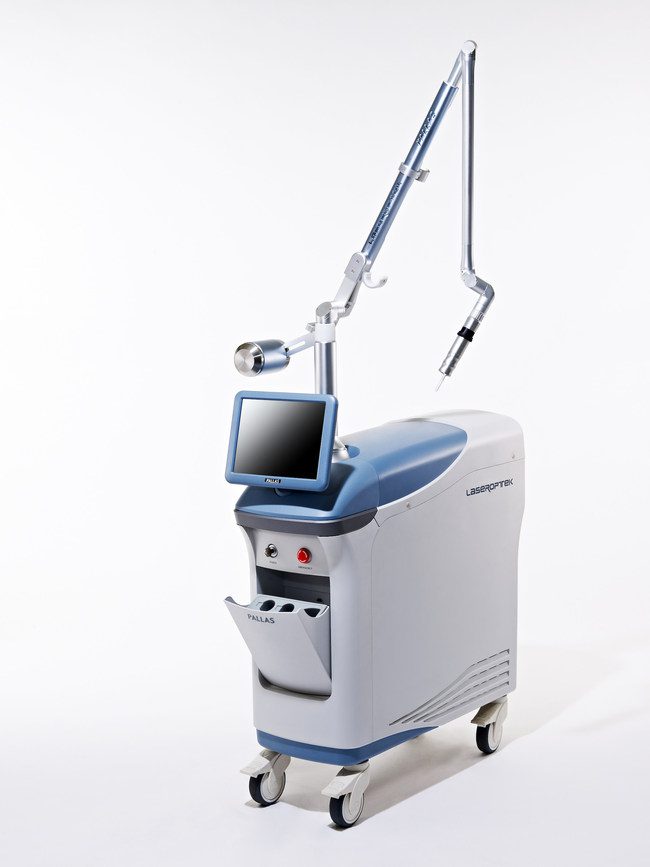
PALLAS Solid-State 311nm UVB laser has been launched in the United States by LASEROPTEK Co., Ltd. The efficacious and safe PALLAS laser is FDA cleared for the treatment of vitiligo, psoriasis, atopic dermatitis, and leukoderma.
PALLAS is the fifth LASEROPTEK laser device to receive FDA clearance in the U.S. for dermatological treatments and brings to the U.S. market the world’s first solid-state UVB laser delivering efficacious and safer treatment for a variety of skin conditions that collectively affect close to a billion people worldwide.
PALLAS features unique technology improvements over 308 nanometer excimer lasers currently used to treat vitiligo, psoriasis, atopic dermatitis and leukodermas. PALLAS provides physicians flexibility by allowing the choice of either 308 or 311 nanometer versions. Both wavelength choices feature PALLAS’ unique, solid-state technology eliminating the need for costly and time-consuming excimer gas and electrode replacements no matter which wavelength is chosen. This reduction in operating costs is a desired and welcomed benefit in today’s challenging market environment.
Dr. Peter Jenkin, owner of Dermatology Associates of Seattle since 2010, is a respected expert in the treatment of pigmentation related skin disorders and is among the first health care providers offering treatments using PALLAS. “I chose LASEROPTEK’s PALLAS UVB laser with the 311nanometer output option given its efficacy and higher safety profile for my patients. I had been aware of research for a few years that the 311nm wavelength might be the best option,” this according to Dr. Jenkin. “I also appreciate the solid-state technology that negates the need for costly and time-consuming service calls to replace electrodes and excimer gas,” added Dr. Jenkin.
Commenting on the launch of PALLAS in the U.S., CJ Lee, CEO of LASEROPTEK said, “LASEROPTEK, in collaboration with our U.S. partners Gale Force Aesthetics and Laser Service Solutions, is deeply committed to developing, selling and servicing advanced laser devices to the U.S. medical and cosmetic dermatology market that improve safety, efficacy and economics for our physician customers and their patients. PALLAS is a great example of this commitment. We very much appreciate Dr. Jenkin’s trust in PALLAS to provide his patients with safe, efficacious and comfortable treatments. We look forward to a long-term relationship with Dr. Jenkin and the entire staff at Dermatology Associates of Seattle.”
Jayson Jonsson, CEO of Gale Force Aesthetics, the exclusive distributor of LASEROPTEK lasers in the U.S. adds, “We are excited and proud to partner with LASEROPTEK to launch PALLAS in the U.S. Physicians need safe and effective treatment options for difficult-to-treat skin conditions like vitiligo, psoriasis, atopic dermatitis and leukodermas. We are confident that this device will provide a much-needed option to physicians and deliver great results for patients.”