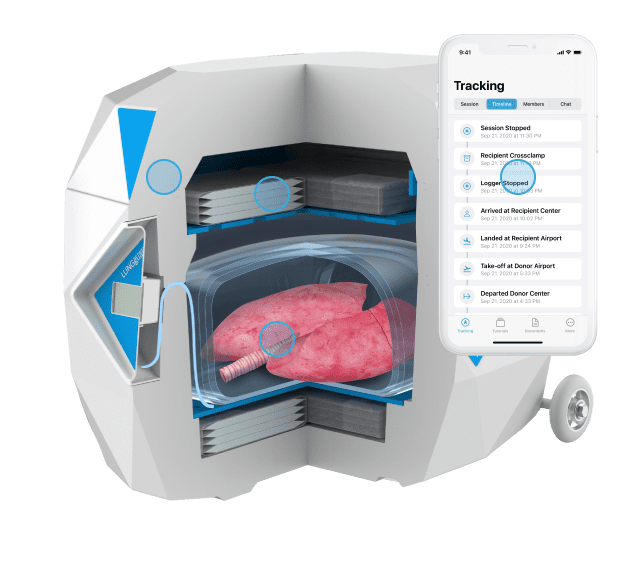
Paragonix LUNGGuard® System: Paragonix Technologies announces a milestone achievement as the device safely completed a record distance for donor lung transport from Alaska to North Carolina.
The longest case in terms of distance and total ischemic time for the LUNGguard Donor Lung Preservation system was completed by Duke University Hospital, one of the largest organ transplant centers in the world.
In the same transplant event, the Paragonix SherpaPak® Cardiac Transport System transported the heart from the same donor in Alaska to a patient in Washington State, with a total ischemic time of over 7 hours and a distance of 1,579 miles. In total, Paragonix Technologies enabled the successful transport of two organs from one donor through a process spanning five flights and 31 hours, resulting in two lives saved by multiple transplant centers across the US.
Four Transplant Centers across the U.S. worked together to make this monumental transplant event happen, including Duke University Hospital, which had a patient in need of a lung transplant. When Duke University Hospital found a donor match in Alaska for its patient, it faced challenges with distance that traditionally would be a high-risk transport due to complications typically associated long ischemic times for organs.
Duke University Hospital contacted Paragonix Technologies requesting a LUNGguard system for use in a long-distance lung transplant case. As the donor was located in Alaska, there was not enough time for the Duke University surgeon team to travel with the system to procure the organ themselves. As a result of this unique situation, Duke utilized a procurement surgeon from a prominent West Coast transplant center, and the Paragonix team worked overnight to transport the LUNGguard Donor Lung Preservation System to that team in time for their flight to Alaska.
“While we consider many factors in selecting a donor, distance is a significant risk factor to moving forward. Transplant centers around the country are seeking ways to overcome the challenges associated with safely transporting organs further and new technologies show promise,” said Dr. John C. Haney, Assistant Professor of Surgery at Duke University
“We are committed to mitigating as much risk as we can in the high-stakes environment of transplantation. This case represents a great example of the possibilities afforded by enabling transplant teams to safely go farther to procure these much need donor organs.” said Lisa Anderson, Ph.D., Founder and CEO of Paragonix Technologies.
Both the Paragonix LUNGGuard System and the SherpaPak Cardiac Transportation System (CTS) provide a sterile and temperature-controlled environment for organs traveling between operating rooms and serve as an alternative to traditional ice transportation. The cooling technology employed by Paragonix Organ Preservation systems ensure thermal protection for up to 40 hours, while providing a rigid and protective structure for physical safety. The devices work with a state-of-the-art app that connect to the Paragonix devices via Bluetooth for on-the-go monitoring, vital for long-distance travel.