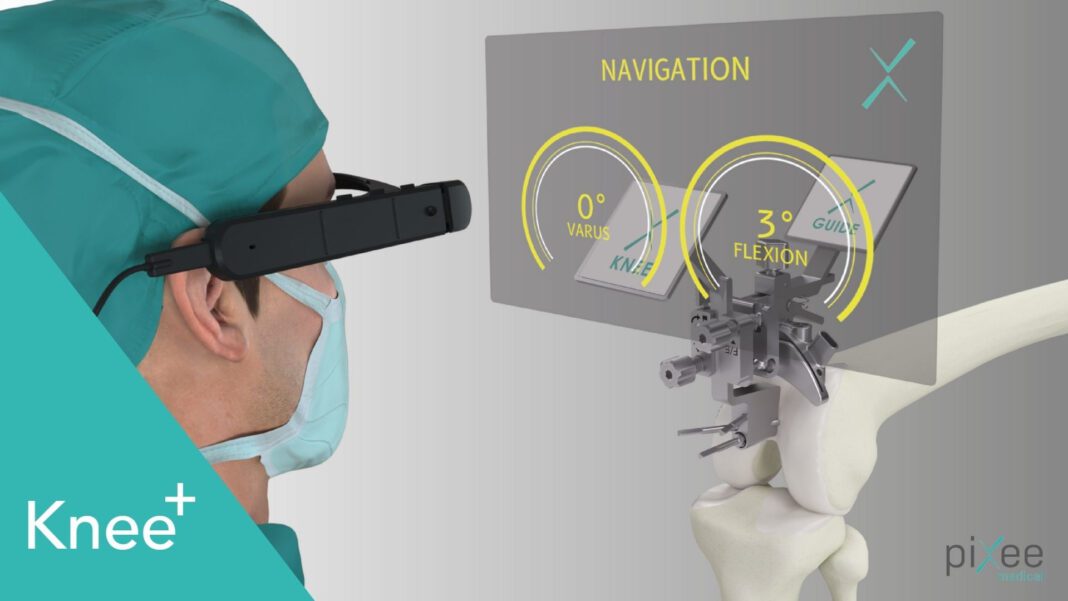
Pixee Medical, a pioneer in augmented surgical guidance technology, has announced that its Knee+ AR computer-assisted orthopedic solution is going to be commercially available in the USA with a perfect fit for Ambulatory Surgical Centers (ASCs) need.
Pixee Medical notes Knee+ is a patented platform designed to help orthopedic surgeons perform surgery through the real-time 3D positioning of instruments, right in their field of view. Knee+ is intuitive and consists of proprietary software using a unique proprietary tracking system running on off-the-shelf smart glasses, with no bulky capital equipment or disposables required.
View video here.
This solution was launched commercially in January 2021 in Europe and Australia. In the challenging COVID context, without any commercial promotion, the product was quickly adopted, with more than 100 systems sold thanks to an easy-to-use and scalable solution.
“Since our first surgery in the USA in Boston in October 2021, we have organized a successful round of surgeries on multiple clinical sites across the USA. We are now ready and confident to launch the product in the leading worldwide market for orthopedics” states Sébastien Henry, Founder and CEO of Pixee Medical. “Our solution is the only imageless & open platform for total knee surgery available in the US.”
“We will be at the AAOS Meeting to meet with surgeons and finalize the organization of our distribution channel.”
Pixee will soon be adding new features to its Knee+ platform, with soft tissue balancing, kinematic alignment and data connectivity. It will also be expanding its portfolio with a mixed reality product for total shoulder arthroplasty and with an easy-to-use cup orientation and leg length controlling AR tool for total hip arthroplasty. Knee+ is now compatible with surgical hoods.