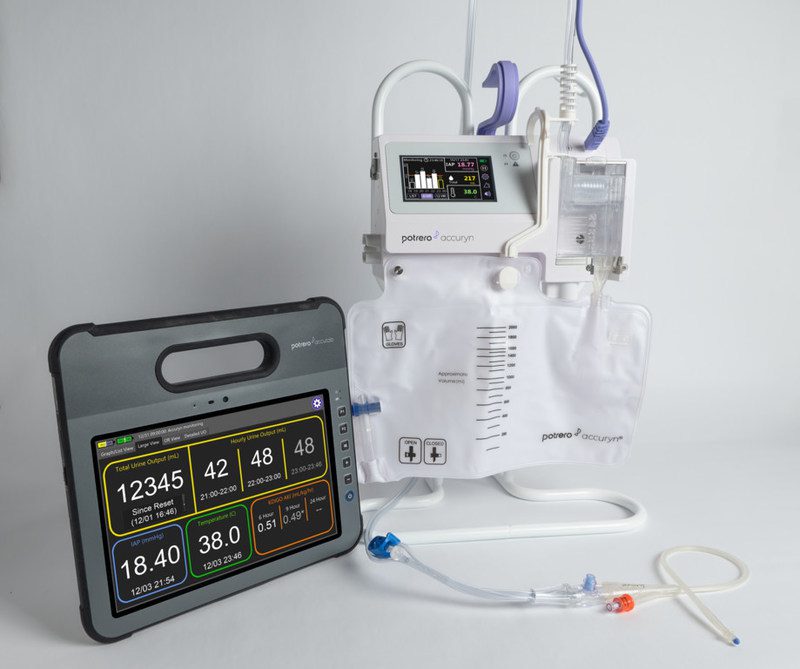
Potrero Medical, innovator of the Accuryn Monitoring System, announced the launch of its newest software App for the Accuryn™ Monitor and AccuTab™. The Accuryn Monitoring System includes a revolutionary sensor-integrated foley catheter that provides real-time, actionable information to healthcare providers, enabling them to take important, earlier steps to prevent disease state progression of critical conditions such as acute kidney injury (AKI).
The app is available exclusively on the Accuryn Monitoring System and will be available starting Monday, November 8, 2021.
“This launch continues our history of innovation and responsiveness to customer needs and is one of several we have planned in our product roadmap for the next several months.” said CEO Joe Urban. “Automating FST is another major step toward our goal of helping clinicians protect the kidney.”
The Furosemide Stress Test (FST) is commonly used in the critical care setting to gauge the likelihood that a patient with early-stage acute kidney injury will progress to more severe stages resulting in major, costly interventions that include readmissions, renal replacement therapy, permanent dialysis and mortality. Traditionally, the FST requires the manual tracking of urine output (UO) over a specific period of time (typically 2 hours), leading to delayed and potentially inaccurate results. The Accuryn Monitoring System now automates the UO tracking portion of the test and logs the result at the exact time prescribed, while advancing workflow for the caregiver team.
In addition to the Furosemide Stress Test, Potrero announced a software upgrade that will launch in unison with the FST App release. The software update enables better patient customization, specifically the ability to enter patient weight down to the 10th of a kg and customize the UO threshold line for each patient.