Pulnovo Medical’s PADN-CFDA clinical trial, the world’s first pulmonary hypertension treatment device RCT study, has completed all patients enrollment and will continue to assist each enrollment center to complete the follow-up of enrolled patients, data recovery, data cleaning, etc., and cooperate with the nation’s research center to complete follow-up work.
According to reports, the PADN device is aimed at pulmonary hypertension and can be used in conjunction with percutaneous pulmonary artery denervation to treat patients with pulmonary hypertension.
Pulmonary hypertension, the occurrence of high blood pressure in the pulmonary arteries, is a disease that is difficult to diagnose and treat. It includes a variety of rare diseases and is called “cancer of the heart and lung vascular system.” Without treatment, the average life span of patients is only 2.8 years. Pulmonary hypertension is a “carcinoma in cardiovascular disease” with a very high incidence and fatality rate. The number of patients worldwide is close to 100 million, but until now, pulmonary hypertension is still one of the lesser-known malignant diseases.
Previously, the existing treatments for patients with pulmonary hypertension mainly relied on targeted drugs and lung transplantation. According to statistics from the US-Canada team in the article Pulmonary hypertension: the unaddressed global health burden published in Lancet Respiratory Medicine in 2018, the cost of drug treatment for patients with pulmonary hypertension is approximately US$80,000 per year. For most patients, this is an impossible expense to bear. As for lung transplantation, due to the limited source of organs and the common complications of infection and rejection in postoperative patients, lung transplantation is extremely difficult, and it is necessary to take anti-rejection drugs for a long time after surgery. In addition, the high cost of surgery also discourages patients.
Pulnovo Medical has brought a new treatment method to patients with pulmonary hypertension. The self-developed radiofrequency ablation product achieved the US FDA Breakthrough designation in 2021. The research results of “Pulmonary Artery Denervation: The New Kid on the Block?” published in the Editorial Comment of JACC Vol. 76, No. 8, 2020 shows that PADN has a better surgical effect and a lower price than drug treatment.
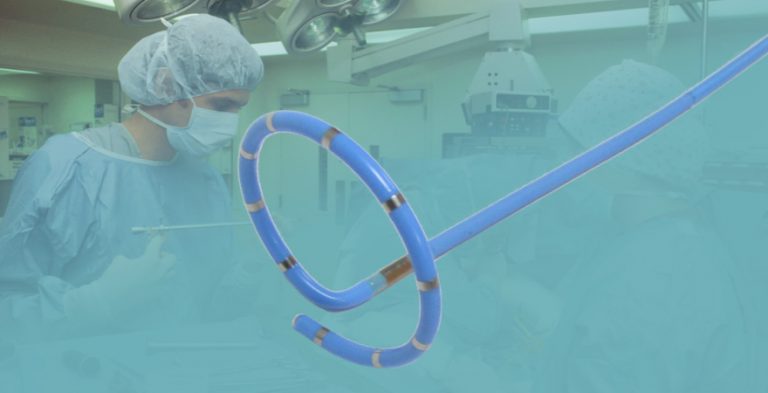
Percutaneous pulmonary artery denervation is an interventional ablation technique that punctures the patient’s femoral vein and sends a special looped catheter to the proximal end of the pulmonary artery bifurcation. After connecting with a radiofrequency ablation instrument, it damages the serous membrane through the intima of the pulmonary artery. The sympathetic nerve under the layer, when the sympathetic nerve of the proximal pulmonary artery is ablated, the pulmonary artery pressure drops.
The clinical data showed that: percutaneous pulmonary artery denervation significantly improved the 6-minute step of polytype pulmonary hypertension, and the mean pulmonary artery pressure and pulmonary vascular resistance were significantly reduced without related complications. In addition, percutaneous pulmonary artery denervation also has a therapeutic effect on mixed pre- and post-capillary pulmonary hypertension secondary to left heart failure.