February 3, 2021
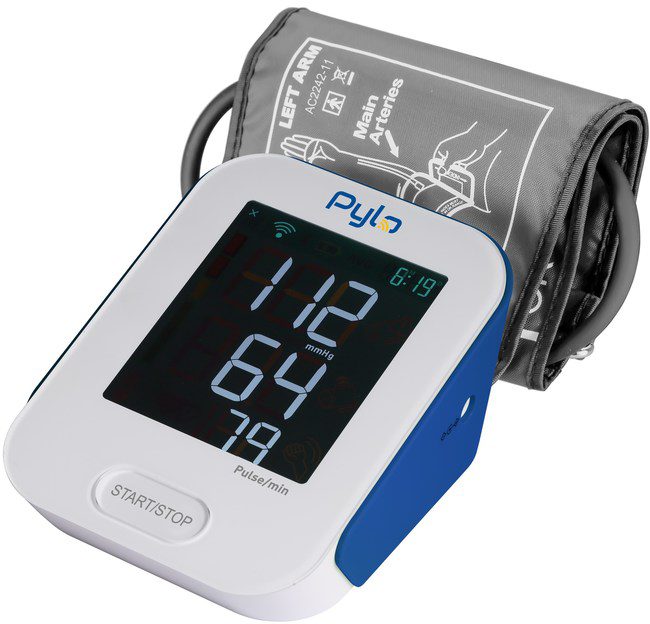
Pylo Health proudly announced today the release of the Pylo 802-LTE, a cellular blood pressure monitor designed for patients undergoing remote physiological monitoring (RPM).
Pylo 802-LTE, from the creators of the Prevounce Platform, is the simplest, most secure way for patients to manage their blood pressure from home. Designed specifically for RPM, it is a highly efficient and cost-effective device for physician practices, health systems, and employer wellness programs working to establish or grow remote patient monitoring programs.
To ensure accessibility for patients of all technical skill levels, the 802-LTE comes fully preconfigured and requires users to press only a single button to use the device. While operating like a traditional blood pressure monitor to patients, the 802-LTE quickly captures and transmits accurate blood pressure and heart rate measurements via encrypted 4G wireless sync to the intuitive Pylo application programming interface (API). The Pylo API then automatically routes the blood pressure measurement data in real time to a user’s doctor, caregiver, family member, or other healthcare organization.
Development of the FDA-cleared and FCC-certified Pylo 802-LTE was prompted by the challenges associated with complex blood pressure monitoring devices, which include difficult setup requirements, inaccurate readings, and awkward user experience. Prevounce Health leadership witnessed these obstacles through the company’s work with provider and health plan clients on the Prevounce Remote Patient Monitoring software.
“Many patients who need to monitor their blood pressure are not tech savvy,” says Daniel Tashnek, founder of Pylo and Prevounce Health. “The configuration and additional steps required for setup and usage of Bluetooth or WiFi monitors represent significant hurdles for those who typically require hypertension management in the first place. The Pylo 802-LTE eliminates these barriers and, more importantly, helps bring the benefits of remote patient monitoring to our most vulnerable populations. Since the 802-LTE was designed specifically for clinical RPM, it has all the privacy and security features expected by patients and providers.”
Additional features include a comfortable cuff, large backlit screen, and an impressive battery life, all of which further help improve patient engagement and participation in remote patient monitoring programs.
“Research has shown that remote cardiac monitoring can greatly reduce patient blood pressure compared to typical care and self-monitoring alone,” Tashnek says. “With ongoing remote monitoring of hypertension via the Pylo 802-LTE, healthcare providers can make more timely recommendations that better support heart-healthy living for their patients.”
The proprietary Pylo Connect API makes it easy for organizations with RPM programs to manage patient readings, connected devices, product ordering, and more in any EMR or third-party care management system. Built with an emphasis on security and HIPAA compliance, the API’s features a fully serverless architecture using Amazon Web Services for scalability, a self-service developer portal, seamless data streaming via event-based webhooks, and a lightweight, RESTful interface.