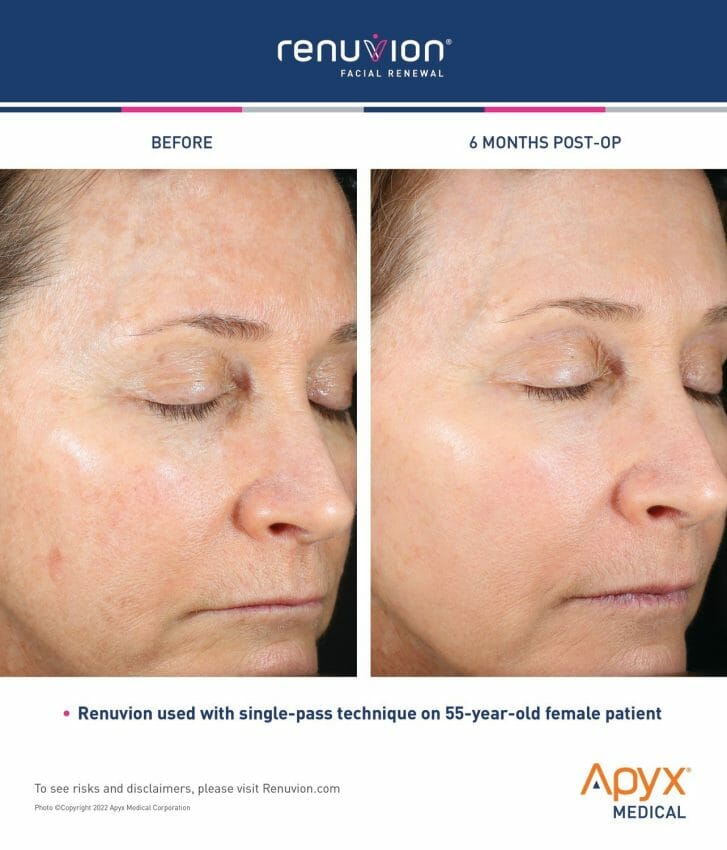
Apyx Medical Corporation (NASDAQ: APYX), the manufacturer of Renuvion®, a proprietary helium plasma and radiofrequency technology, previously announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for the use of the Renuvion Dermal handpiece for specific dermatological procedures for the treatment of moderate to severe wrinkles and rhytides, limited to patients with Fitzpatrick skin types I, II or III.
Branded as Facial Renewal, this treatment for wrinkle-reduction provides patients with an effective and nonsurgical option for smoothing and contracting the skin in one procedure. Facial Renewal enables dermatologists, plastic surgeons and cosmetic physicians to achieve transformative results, without the patient needing invasive surgery or risking looking unnatural. Facial Renewal with Renuvion is the first major advancement in decades of skin treatments for the face, providing patients with dramatic and natural-looking results.
“Renuvion for facial procedures is groundbreaking. It’s a treatment that fulfills a big unmet need in cosmetic procedures,” said New York oculoplastic surgeon Tabasum Mir, MD. “I have been using the Renuvion technology on patients in my practice and I’ve seen the transformative results firsthand. With the new clearance for Facial Renewal, this opens a whole new treatment option for patients to achieve the youthful appearance they’re seeking.”
“We are very pleased to receive FDA clearance with a specific clinical indication that enables Apyx Medical to market and sell our Renuvion Technology to physicians and patients for use in dermal resurfacing procedures,” said Charlie Goodwin, President and Chief Executive Officer. “Facial Renewal with Renuvion offers patients a refreshed and more youthful look without having the unnatural ‘pulled’ appearance often associated with a facelift.”