Abiomed (Nasdaq: ABMD) announces the results of the Restore EF study demonstrate Impella-supported high-risk percutaneous coronary intervention (PCI) leads to significant improvements in left ventricular ejection fraction (LVEF), angina symptoms and heart failure symptoms at follow-up. The study, which published online August 12 in JSCAI, builds on the largest clinical data set ever collected for high-risk PCI and further validates the LVEF and quality of life benefits associated with Impella-supported procedures.
Restore EF is a prospective, multi-center study evaluating the best practices in contemporary PCI practice, including more complete revascularization. Participants received an Impella-supported high-risk PCI, also called an “on-pump PCI,” at one of 22 sites across the United States between August 2019 and May 2021. At 90-day follow up, study participants had:
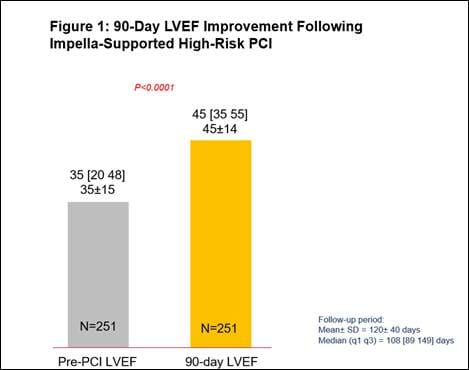
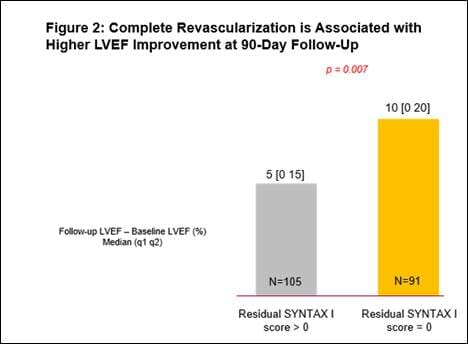
- A 29% relative improvement from baseline LVEF (n=251, p<0.0001), with a significantly greater improvement in LVEF for those who had a complete revascularization (characterized by a residual SYNTAX score of 0). (See figures 1 and 2)
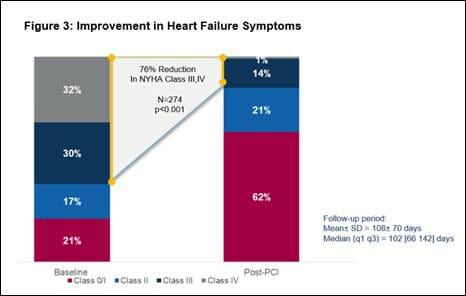
- Significant improvement in heart failure symptoms, with an overall 76% reduction in New York Heart Association Class III or IV heart failure symptoms (n=274, p<0.001). (See figure 3)
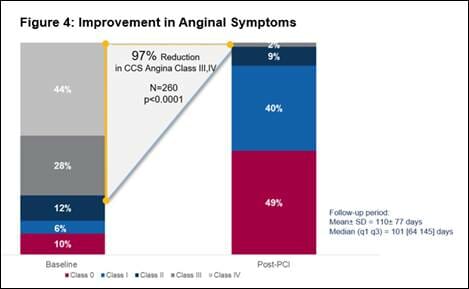
- Significant improvement in angina symptoms, with an overall 97% reduction in Canadian Cardiovascular Society Class III or IV angina symptoms (n=260, p<0.0001). (See figure 4)
Participants with higher baseline LVEF (greater than 45%) also experienced significant symptomatic improvement, similar to patients with lower LVEF.
“The Restore EF study results add to the growing body of evidence demonstrating that Impella-supported high-risk PCI can lead to a more complete revascularization and considerable LVEF improvement,” said Jason Wollmuth, MD, Restore EF study principal investigator and an interventional cardiologist at Providence Heart Institute. “The improvement in angina and heart failure symptoms in those with near normal LVEF provides a clear benefit to this patient population that is aligned with the FDA’s 2018 decision to expand Impella’s indication to patients undergoing high-risk PCI with or without depressed ejection fractions.”
An accompanying editorial published in JSCAI, written by physicians from Massachusetts General Hospital and Harvard Medical School, concludes, “based on the encouraging report from Wollmuth et al, a heart team–based recommendation for PCI in CABG-ineligible patients appears reasonable for patients with ICM (ischemic cardiomyopathy).”
Results from Restore EF and the PROTECT III study, which published in the June 2022 edition of the American Heart Journal, further demonstrate the safety and benefits of Impella-supported high-risk PCI, including low bleeding and MACCE rates (composite of death, stroke, myocardial infarction and repeat procedures). Data from PROTECT III shows reduced MACCE rates compared to PROTECT II (15.1% vs. 21.9%, p=0.037) when Impella is used to achieve a more complete revascularization in a single setting for high-risk PCI patients. Operators from community and academic centers demonstrated low major bleeding rates of 2.5% in the Restore EF study and 1.8% in the PROTECT III study when contemporary best practices were followed.
“These results further demonstrate that high-risk PCI procedures supported by Impella employing contemporary best practices are safe and effective, providing a treatment option for patients who have historically had limited options to improve their quality of life,” said Mitul Patel, MD, the study’s first author and an interventional cardiologist at UC San Diego Health.
Restore EF and PROTECT III are the latest in a growing list of studies that demonstrate Impella-supported high-risk PCI leads to improvement in LVEF:
- Journal of the American College of Cardiology, 2009 – The PROTECT I trial found patients who had a Protected PCI with Impella had a 31% improvement in LVEF at 30-day follow up. (From 26 ± 6% to 34 ± 11%, p=0.003).
- Catheterization and Cardiovascular Interventions, 2011 – This study, led by Maini, et al., found a 17% improvement in LVEF at follow up, after a Protected PCI with Impella (p<0.0001).
- Circulation, 2012 – The PROTECT II randomized controlled trial found Protected PCI with Impella led to a 58% improvement in NYHA Class III and IV heart failure symptoms at 90 days (p<0.001). The trial also found, during follow up after Protected PCI with Impella, patients had a 22% improvement in LVEF (p<0.001).
- Journal of Interventional Cardiology, 2013 – This study, led by O’Neill, et al., suggests that early initiation of hemodynamic support prior to PCI with Impella 2.5 is associated with more complete revascularization and improved survival to discharge compared to post-PCI support (65.1% vs. 40.7%, p<0.003).
- American Journal of Cardiology, 2013 – An analysis of the PROTECT II randomized controlled trial by Dangas, et al., found Impella use led to a 29% reduction in major adverse cardiac and cerebrovascular events (MACCE) at 90 days, compared to the use of the intra-aortic balloon pump (IABP) (p=0.042).
- Journal of Interventional Cardiology, 2019– This study, led by Burzotta, et al., found six months after a Protected PCI, the percentage of patients with LVEF greater than or equal to 35% increased by 205%, from 22% to 67% (n=79, p≤0.001). The study also found more complete revascularization was associated with significant LVEF improvement and survival.
These studies have led to the PROTECT IV randomized controlled trial, which began enrolling patients in April 2021. PROTECT IV compares on-pump PCI to off-pump PCI and is the final step on the clinical evidence pathway to a Class I recommendation for Impella use in high-risk PCI. PROTECT IV leverages the best practices that physicians have learned during the past 10 years of clinical studies that led to the exclusive FDA PMA approval of Impella for high-risk PCI.
Additional information about the Restore EF study, including case studies and interviews with the study’s authors, is available on HeartRecovery.com.