Robocath, a company that designs, develops, and commercializes cardiovascular robotic platforms for the treatment of vascular diseases, today announces the successful completion of the first five robotic coronary angioplasties in Belgium.
The Percutaneous Coronary Interventions (PCI) were performed on April 8 and 13 by Prof. Stefan Verheye, a recognized and highly experienced interventional cardiologist at ZNA* Middelheim hospital in Antwerp, and his team. Robotic-assisted PCI has never been done before in this country.
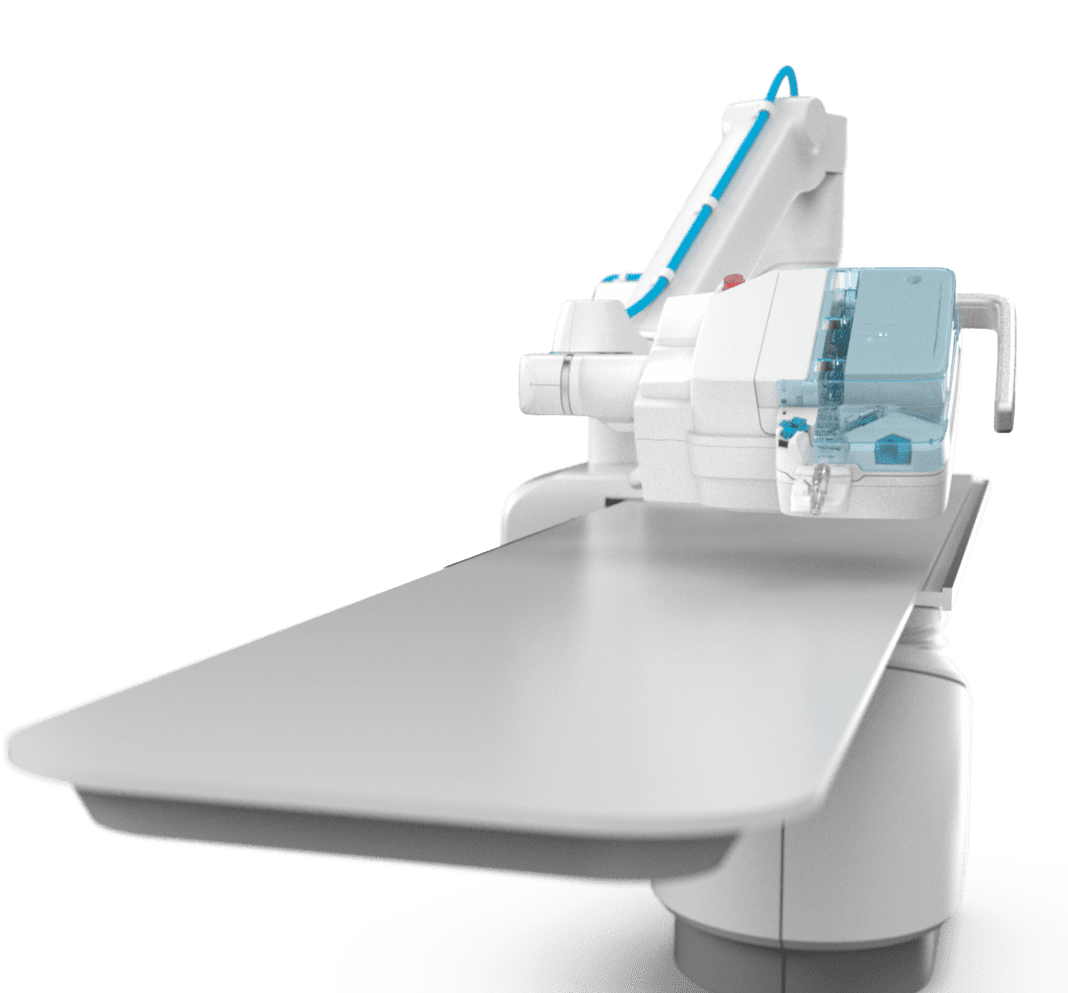
R-One is a robotic platform that assists interventional cardiologists in performing coronary angioplasties. This procedure consists of revascularizing the cardiac muscle, by inserting one or more implants (stents) into the arteries that supply it with blood.
R-One is the first solution developed by Robocath. This robotic platform is designed to operate with precision and perform specific movements to facilitate and enhance the interventional procedures performed on the patient. It also offers a better working environment for physicians and the entire medical team, with a drastic reduction in X-ray exposure.
These first five robotic procedures are part of a clinical study that involves six European centers. The study aims to demonstrate the many benefits for interventional cardiologists of robotic use within daily practice.
Prof. Stefan Verheye, Interventional Cardiologist at ZNA Middelheim, Antwerp, member of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and author of over 170 peer-reviewed papers (mainly investigating new approaches and innovative technologies), said: “I’m honored to be the first user in Belgium of this French vascular robotic solution. I was immediately very impressed by the ease of use of the platform and its level of precision. The robotic arm places the stent even more precisely than with the manual technique, down to a fraction of a millimeter. In addition, it allows us to work in a much safer and more comfortable setting because we are no longer directly in contact with harmful X-rays – which is of great concern to all physicians in this field. It also means that we no longer need to wear our lead apron which can often cause orthopedics injuries. Therefore, I performed five clinical robotic procedures in a comfortable seated position, without any X-ray exposure. This robotic solution is the first step toward a global revolution in interventional cardiology. I have absolutely no doubt that this solution will be widely taken up. I’m delighted to be among the pioneers of this new technological wave in Europe.”
*Hospital Network Antwerp (Ziekenhuis Netwerk Antwerpen- ZNA) is the largest healthcare organization in Belgium. Within its ten sites, the group comprises of three general hospitals, six specialized hospitals, a psychiatric nursing home and a residential care center. ZNA’s sphere of influence extends to over 32 municipalities in Belgium – potentially reaching more than a million inhabitants. In the city of Antwerp, ZNA achieves a patient share of 44 percent.
ZNA employs about 6,300 people, including 600 doctors. In total, ZNA has approximately 2,500 beds. Every year, more than 50,000 people are hospitalized (admitted with an overnight stay) and more than 70,000 patients receive treatment in a day clinic. The number of outpatients is around 600,000 per year.