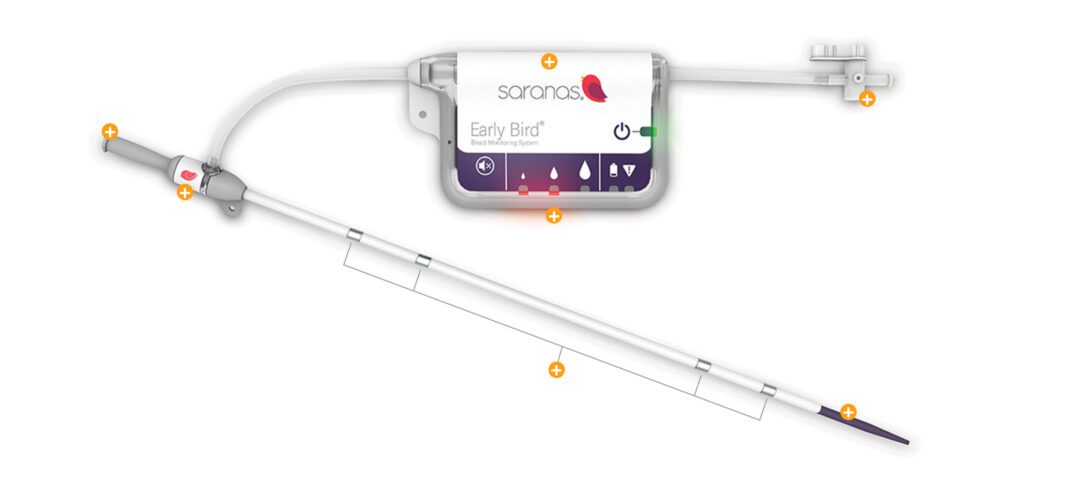
Saranas, a company focused on improving patient outcomes through early detection and monitoring of internal bleeding complications, announced today that the U.S. Department of Commerce’s United States Patent and Trademark Office (USPTO) has issued them Patent No. 11,224,414.
Titled “Access Closure with Bleed Monitoring,” this key patent allows for embedding a vascular access closure device with the company’s proprietary bleed monitoring technology. Saranas’ Early Bird Bleed Monitoring System® is the first and only FDA-approved device for real-time detection and monitoring of endovascular bleed complications.
“As we continue to grow our commercial presence with the Early Bird, we are pleased to secure this important patent that is designed to further expand the implementation of our differentiated bleed monitoring technology,” said Saranas CEO James Reinstein. “This patent award demonstrates Saranas’ commitment to innovation and further strengthens our intellectual property portfolio.”
“From vessel injury to failure of obtaining hemostasis with access closure devices, bleeding complications in interventional procedures continue to pose challenges for clinicians,” said Dr. Timothy Henry, Lindner Family Distinguished Chair in Clinical Research and Medical Director of The Carl and Edyth Lindner Center for Research at The Christ Hospital. “Technologies to better manage access site complications are paramount as the continued emergence of percutaneous interventions often require large-bore vascular access.”
Dr. Philippe Généreux, interventional cardiologist, commented, “We have been using the Early Bird in our clinical practice for the past two years, and the current design of incorporating a fully functional introducer sheath with bleed detection allows for seamless integration into high-risk interventional cardiovascular procedures. Embedding bleed detection directly onto a vascular closure device is the eventual next step and has the potential to become the standard of care across all types of vascular access procedures.”