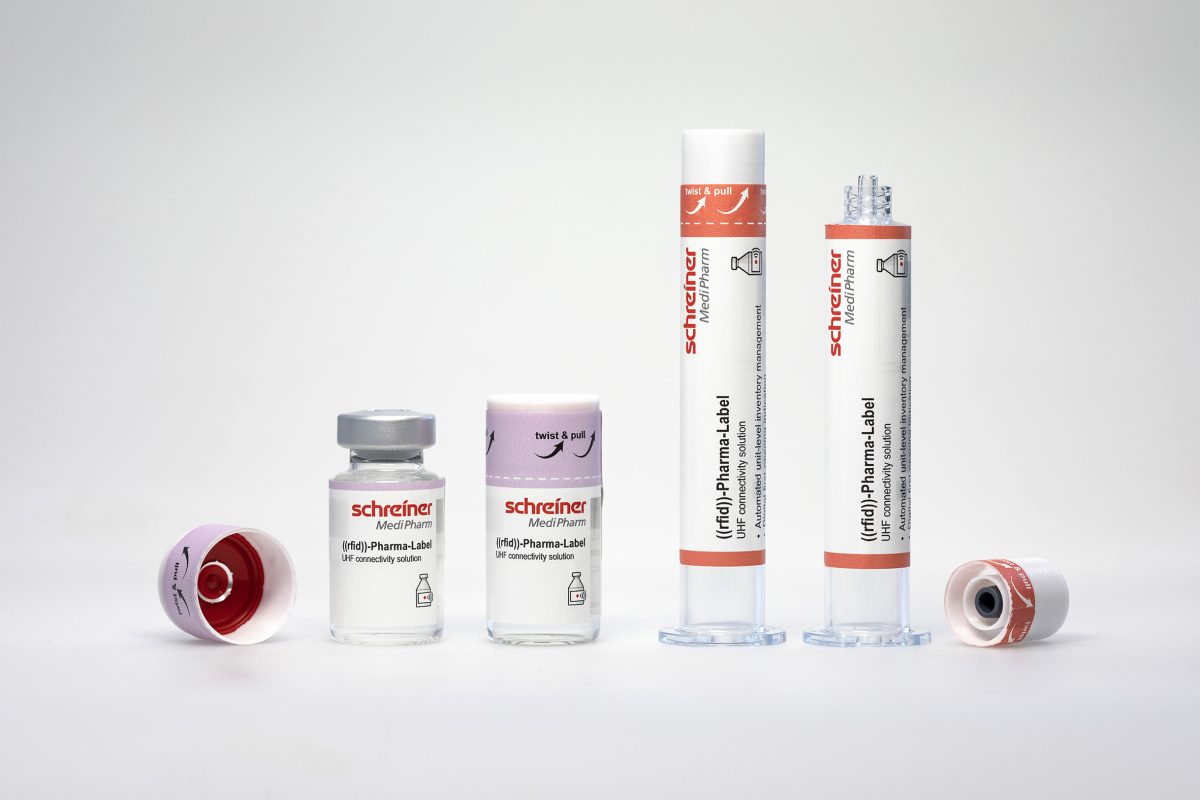
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, will showcase its new RFID label with digital first-opening indication at Pack Expo 2024, November 3-6 in Chicago. The new solution – which irreversibly indicates a container’s initial opening, and enables automated integrity tracking – will be among those displayed at the company’s Booth W-16094.
Digitization and process automation are increasingly sought-after in healthcare settings, as such setups increase product safety and avoid medication errors. Labels with integrated RIFD chips can play an important role, since they are used for product marking while simultaneously enabling unique unit-dose digital IDs. This is a decisive prerequisite for complete end-to-end tracking of containers within the supply chain. However, in hectic hospital settings it must also be ensured that the integrity of prefilled syringes or vials has not been compromised prior to medication administration.
Schreiner MediPharm’s new RFID label comprises an integrated digital sealing function whose design irreversibly indicates a container’s initial opening, and enables automated integrity tracking. This eliminates the need to manually check whether the container has already been opened. This not only makes work easier for medical staff, but also optimizes inventory management, since unused medication can be efficiently returned to stock. At the same time, possible diversions and medication misuse can be monitored.
Schreiner MediPharm’s new label features a special design that reliably protects the integrated chip from mechanical stress and impact during the pharmaceutical production process; this buffering effect can pay particular dividends for manufacturers utilizing high-speed dispensing systems. The result is a digital seal that can be reliably read from production to final application – an important aspect in ensuring seamless unit-level medication tracking for increased product and patient safety.
Also at Pack Expo, Schreiner MediPharm will highlight recently introduced label solutions for applications with sensitive APIs, as well as its latest sustainability-minded product solutions. For example, the company’s Needle-Trap Secu combines needlestick safety elements with an integrated first-opening indication – a setup that eliminates the need for an additional blister pack, resulting in considerable space savings as well as reduced waste and cost.