SentiAR, Inc. a St. Louis, Missouri based pioneer in using Augmented Reality (AR) visualization technology for medical procedures, has closed an $8.5 million Series B financing. The financing was led by cultivate (MD) Ventures and joined by MedVenture Partners alongside several insider investors, including TechWald Holding, VCapital, QRM Capital, and Harmonix Fund. The funding will allow the company to launch additional partnerships and wider clinical deployments.
View video here.
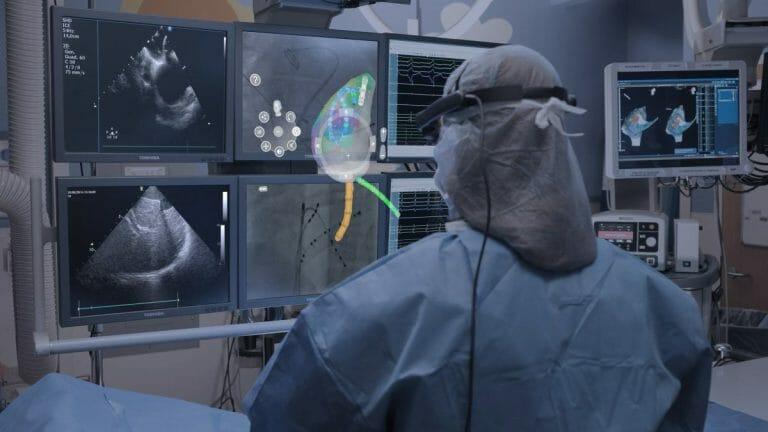
The company’s innovative first product, CommandEP™, integrates existing imaging systems to create a real time 3D holographic interface. This interface provides physicians with an interactive, 360-degree view of the patient’s specific anatomy to allow them to deliver cardiac ablation therapy to patients. The CommandEP system utilizes a specially designed headset worn by the physician allowing hands-free manipulation of the patient’s data for the first time, in addition to real-time heart anatomy and catheter locations inside the heart. The CommandEP system is designed to be utilized during cardiac ablation procedures to increase physician navigation accuracy and speed. This technology offers physicians control of their digital tools and has been shown to improve physician navigation accuracy in the heart by up to 50%.
Berk Tas, CEO of SentiAR said, “We are delighted to welcome new investors. This funding allows us to meet the great interest from physicians for this first of its kind application. We look forward to working with our clinical and strategic partners as we scale up clinical deployments.”
Dr. Jag Singh, MD, PhD, Cardiac Electrophysiologist, Professor of Medicine at Harvard Medical School, and Founding Director of Resynchronization and Advanced Cardiac Therapeutics Program at Massachusetts General Hospital Heart Center, said, “We have been looking for a tool like this for a long time. The CommandEP system’s ability to provide full 3D visualization in real-time and giving us the ability to control our images, hands-free, for the first time is exciting. We look forward to the potential benefits of CommandEP for our patients.”
Dr. R. Sean Churchill, MD, MBA, Executive Director of Genesis Innovation Group’s cultivate (MD) Ventures said, “Our group has been looking for a valuable clinical AR solution that has the potential to disrupt how clinical procedures are performed. SentiAR’s, CommandEP system fits this perfectly. It is profoundly impactful, exceptionally simple and does not impede clinical workflows. The system is developed by a star team. We are proud to partner with them and bring CommandEP to serve our physicians and patients.”
CommandEP is the first application of its kind in the world and SentiAR anticipates completion of new clinical studies in the second half of this year.