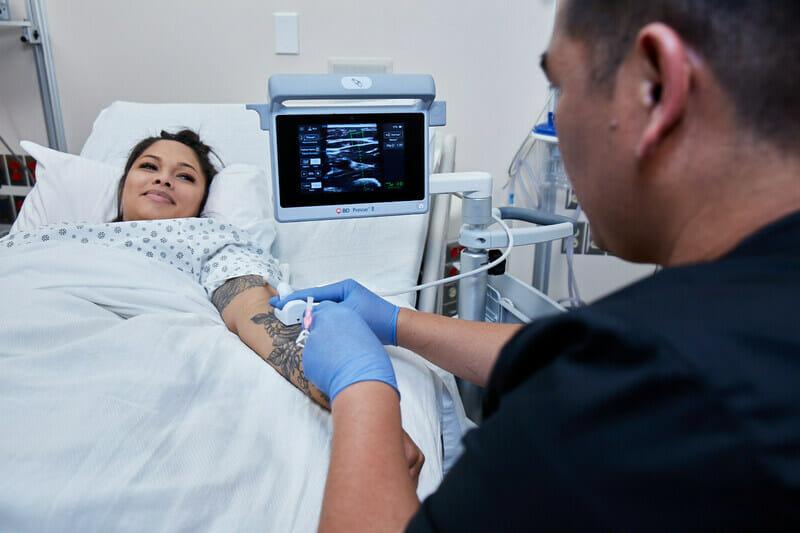
This new, easy-to-use advanced ultrasound device offers a specialized probe designed to provide clinicians with optimal IV placement.
BD Introduces Advanced Ultrasound Technology to Help Drive First-Stick Success for IV Insertions
Linkedin
Twitter
WhatsApp
Facebook
Copy Link
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today launched a new, easy-to-use advanced ultrasound device with a specialized probe designed to provide clinicians with optimal IV placement.
The BD Prevue™ II System addresses an unmet need in IV access through real-time needle depth markers. The system features the BD Cue™ Needle Tracking System, offering a high-quality ultrasound image of the needle trajectory, and is compatible with BD Cue™ Needle Tracking-enabled catheters. Simulated studies show that pairing a needle-tracking system with ultrasound guidance may help reduce the number of attempts and time to successful vessel access — which, according to the American Institute of Ultrasound in Medicine, may make vascular access procedures safer and easier for clinicians and patients.1
More than 90 percent of hospitalized patients receive IV therapy through a peripheral IV catheter2 — representing hundreds of millions of patients a year.3 However, nearly two-thirds4 of these patients have vessels that are difficult to visualize and access, often resulting in additional needlesticks, vessel damage and infiltration into the surrounding tissue.
“Difficult IV access remains far too common today, but the availability of the BD Prevue™ II System supports a future where every needlestick has a predefined pathway for successful placement,” said Eric Borin, worldwide president of Medication Delivery Solutions at BD. “BD is advancing the vision of a ‘One-Stick Hospital Stay,’ and first-stick success is the first requirement to fulfill that vision. First-stick success also reduces the pain and anxiety patients often experience while undergoing multiple IV access attempts and provides clinicians with IV workflow efficiencies and confidence.”
The system is designed with clinician-friendly features to help expand the use of ultrasound in IV placement. For example, the BD Prevue™ II System offers a specialized probe designed to help reduce the learning curve — ensuring that clinicians can use the technology without changing their current insertion technique or field of view.
“Patients living with chronic conditions like heart failure may suffer from poor circulation and large amounts of scar tissue from frequent venous access making their veins more difficult to locate and creating a challenge for placing a good, lasting peripheral IV line,” said Lisa Wilson, registered nurse and night shift lead for the progressive cardiac care unit at INTEGRIS Health Baptist Medical Center in Oklahoma City, Okla. “With the use of ultrasound guidance technology, we are able to quickly and successfully place IVs in our hard-to-stick patients, leading to quicker treatment and fewer sticks for our patients and a better experience for everyone involved.”
As the global leader in vascular access solutions, BD is committed to advancing the standard of care for IV therapy and blood draws for patients and health care providers. The availability of the BD Prevue™ II System further drives the BD “One-Stick Hospital Stay” vision to help reduce unnecessary needlesticks by choosing the right vascular access device and placing it successfully the first time. This is one of three pillars of the vision that also includes using one IV line as a single access point for required therapies and blood draws, and optimal maintenance of the IV line to help reduce the risk of complications so it does not have to be replaced and lasts throughout a patient’s hospital stay.
| 1 Alsbrooks, K, Hoerauf, K. Comparative Effectiveness, Efficiency, and ED Nurse Preference Between Two Methods of Visualization for Midline Catheter Insertion: A Pilot Study. SAGE Open Nursing. 2023. |
| 2 Bahl, A, Hijazi, M, Chen, N, et al. Ultralong versus standard long peripheral intravenous catheters: A randomized controlled trail of ultrasonographically guided catheter survival. Anals of Emergency Medicine. 2020;76(2):134-142 |
| 3 iData Research Inc. US Market Report Suite for Vascular Access Devices and Accessories. 2020. |
| 4 Armenteros-Yeguas, V, Garate-Echenique, L, Tomas-Lopez,MA, et al. Prevalence of difficult venous access and associated risk factors in highly complex hospitalized patients. Journal of Clinical Nursing. 2017;26:4267-4275 |