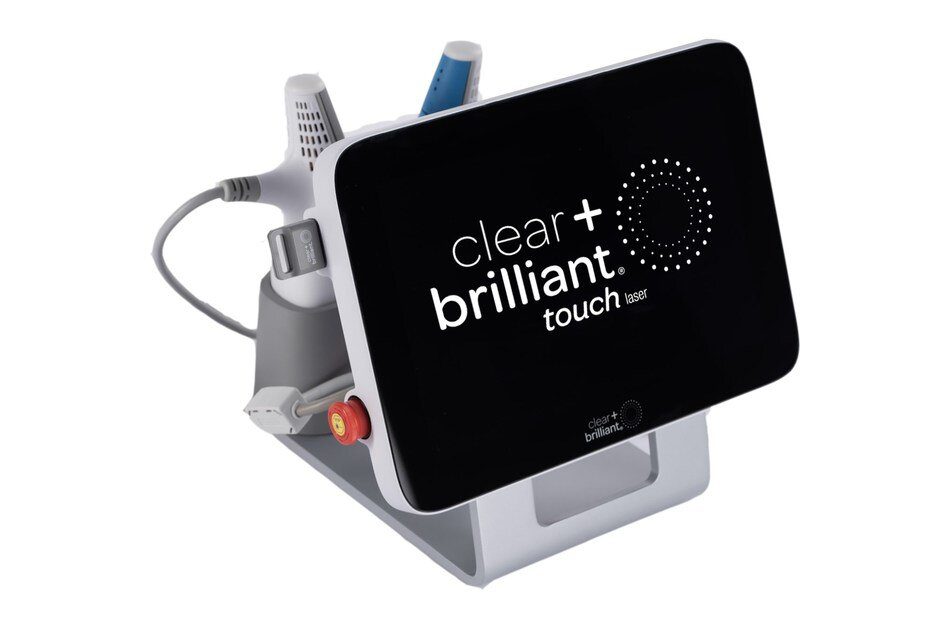
Solta Medica*l notes the Clear + Brilliant Touch Laser delivers a customized and more comprehensive treatment protocol by providing patients of all ages and skin types the benefits of two wavelengths.
By seamlessly switching between two wavelengths with the Original and Perméa® handpieces, physicians can now more easily deliver a more complete treatment during a single appointment.
“The new innovative features of the Clear + Brilliant® Touch laser are designed to make the system easier and more convenient to use. Aesthetic providers can now more efficiently offer patients a complete treatment using two wavelengths by seamlessly switching between the Original and Perméa® handpieces during one appointment. We believe physicians and their patients will experience a real advantage with the new Clear + Brilliant® Touch laser,” said Tom Hart, global vice president, Solta Medical. “As pioneers in the aesthetic market, Solta Medical has continued to evolve the Clear + Brilliant® technology to improve upon the efficiency and convenience that physicians and their patients have come to expect from Solta Medical.”
In addition to offering easy access to both the Original and Perméa® handpieces in the compact system, the Clear + Brilliant® Touch laser also includes a user-friendly touchscreen, a simple training mode to simulate treatment without emitting laser energy and a smart charting assistant that automatically tracks treatment progress with the ability to export patient treatment charts.
“The Clear + Brilliant® laser is an easy-to-use and transportable system that has been a mainstay in my practice for years. The new system, the Clear + Brilliant® Touch laser offers even more versatility, for my patients. Having the option to use both wavelengths seamlessly during one appointment will be a welcome addition to my practice and to my patients,” said Jeffrey S. Dover, M.D., FRCPC, a board-certified dermatologist in Boston, MA.
*Solta Medical, part of the Ortho Dermatologics business of Bausch Health Companies Inc., is a global leader in the medical aesthetics market that helps drive revenue growth to aesthetic practices by providing innovative and effective skin rejuvenation and body contouring solutions. Solta Medical’s vision is to develop and support trusted aesthetic brands that provide value and lasting growth to physicians and patients. These include the Thermage® RF systems, Fraxel® laser, Clear + Brilliant® laser and VASER® ultrasonic systems. More than six million procedures have been performed with Solta Medical’s portfolio of products around the world.