February 11, 2021
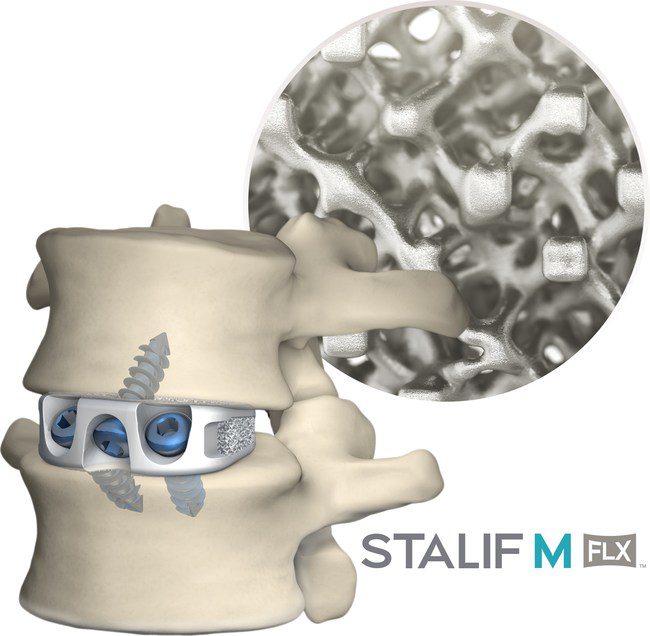
STALIF M FLX platform represents the latest material advancement offered by Centinel Spine.
FLX implants are 3D-printed porous titanium, meticulously engineered down to the cellular unit level to mimic bone. The FLX family of implants boasts equivalent subsidence performance to PEEK; contains a proprietary, interconnected FUSE-THRU™ lattice with a structure similar to bone; and has an optimized mechanical environment to reduce stress shielding, enable fusion assessment, and support bony in-growth, on-growth, and thru-growth.
STALIF M FLX implants have been engineered based on the STALIF® design which has over 30 years of proven clinical history and has helped thousands of patients regain their lives—including the world’s winningest athlete, Tiger Woods. STALIF implants uniquely provide compressive fixation at the fusion site, pulling the vertebral bodies onto the implant and graft material to enhance opportunities for fusion in line with Wolff’s Law of Bone Healing.
“Centinel Spine is proud that the STALIF M FLX device has been recognized as the top ALIF 3D-printed cage,” stated Centinel Spine CEO Steve Murray. “As a company, we remain dedicated to continued product quality, innovation, and furthering clinical evidence. STALIF M FLX is the latest evolution of the STALIF portfolio, and continues our heritage in advancing the stand-alone market founded over 30 years ago,” concluded Murray.