March 11, 2021
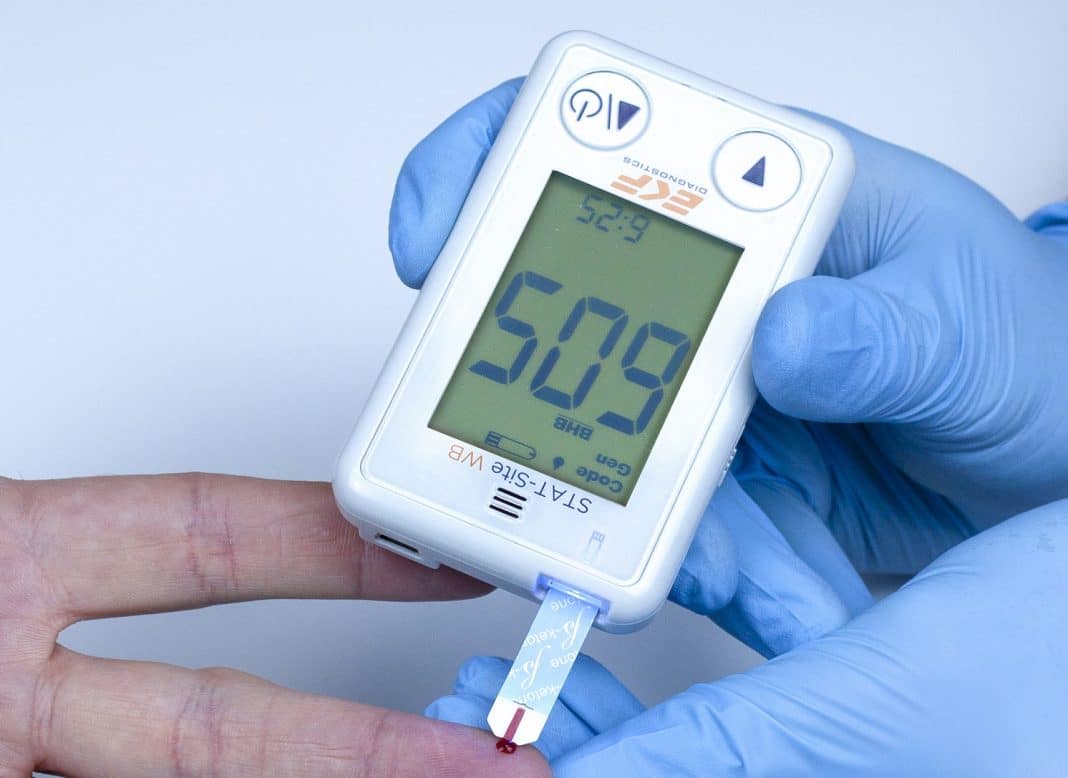
STAT-Site WB handheld analyzer delivers β-ketone and glucose measurements from whole blood in seconds.
This follows the securing of its CE mark certification alongside its FDA CLIA-waiver, meaning that the portable, dual-purpose analyzer is ideal for professional use in the management of diabetes patients.
The STAT-Site® WB is used for the quantitative determination of β-ketones (Beta-Hydroxybutyrate or BHB) and glucose in whole blood taken from capillary or venous samples.
The easy-to-use analyzer is reagent free, utilizing two different test strips to deliver results in just 10 seconds for β-ketones and 5 seconds for glucose. This enables clear and comprehensive management of diabetes patients displaying early symptoms of ketosis, ensuring their rapid treatment and subsequent regular monitoring.
βHB is used to aid the diagnosis and monitoring of patients with ketosis (diabetic ketoacidosis or DKA). As β-HB accounts for 78% of all ketones present in the body, and is the first to appear during ketosis, it provides the clearest indication of a patient’s condition before and after treatment.
The STAT-Site WB is calibrated to the gold standard Liquicolor Beta-Hydroxybutyrate method, meaning that it delivers laboratory accurate results within seconds near to the patient.
Further ensuring results accuracy, the handheld analyzer is simple and intuitive to use, even for inexperienced users. The STAT-Site WB auto-starts when a test strip is inserted and will also automatically detect if it is a BHB or glucose test to be undertaken.
Just 1 µL of whole blood is required for direct analysis and the system will also detect and alert if insufficient blood is available. An automatic strip ejection function ensures that cross-contamination is avoided.
Also making it ideal for remote testing, the analyzer uses two 1.5V AAA alkaline batteries which are easy to obtain and replace, whilst test strips are available individually foil wrapped or in a vial with a shelf life of six months once opened. Results are clearly displayed on a large LCD screen interface with a capacity for up to 400 measurements to be stored on the system – these can be downloaded via a mini USB lead.
“STAT-Site WB provides an affordable, easy-to-use technology that can help to reduce the cost of long-term healthcare of the growing diabetic and pre-diabetic population globally,” said Gavin Jones, Head of Product Management, EKF Diagnostics. “Having direct access to lab-quality β-ketone and glucose near-patient results ensures the practitioner has the essential data needed to make informed clinical or lifestyle decisions in seconds.”