Stratasys Ltd. (NASDAQ: SSYS), a leader in polymer 3D printing solutions, today announced that it is sponsoring the University of Minnesota Visible Heart® Laboratories with a donation of Stratasys J750™ Digital Anatomy™ 3D printers, as well as MakerBot METHOD X® and MakerBot SKETCH® 3D printers.
The Visible Heart Laboratories are a large research lab located at the University of Minnesota Medical School that supports medical research, student and physician education, and medical device product development and testing.
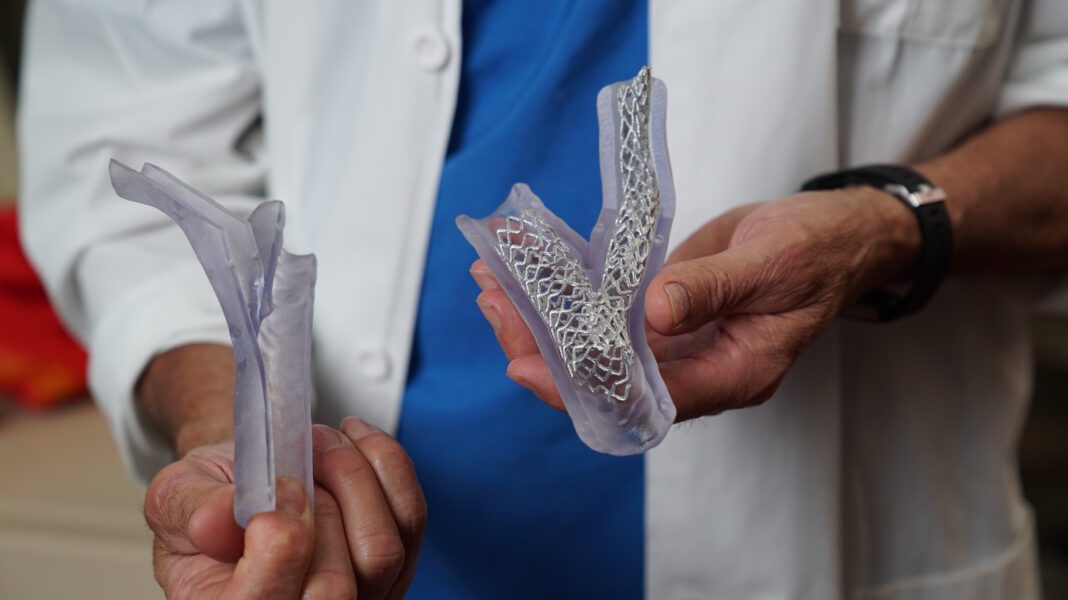
Stratasys’ Digital Anatomy 3D printers produce anatomic models that mimic the actual feel, responsiveness, and biomechanics of human anatomy. Models can be punctured, sutured, cut, and physically manipulated like actual human tissue. These attributes are especially important to the education, research and product development conducted in the Visible Heart Laboratories.
During their education, all University of Minnesota medical students pass through the Visible Heart Laboratories to learn about the benefits of 3D printing technology and how anatomic models can assist them in their future practice. The Visible Heart Laboratories also are committed to providing outreach education about cardiac anatomy and device therapy to students from middle school age to clinical cardiologists.
“The Visible Heart Laboratories are driven to train the next generation of medical device developers and provide them with the abilities to 3D print prototypes and/or virtually placed devices within real heart anatomies, as today these are considered essential skills,” said Dr. Paul Iaizzo, professor Visible Heart Laboratories, University of Minnesota Medical School.
In addition to student education, physicians at the M Health Fairview University of Minnesota Medical Center utilize 3D printing technology to educate patients with anatomic models that represent a patient’s actual anatomy and pathology. Anatomic models can reflect an individual patient’s demographics, comorbidities, or anatomic details like the accumulation of plaque in an artery or the presence of tumors. With these models, the medical team can easily describe treatment and surgical options with patients, leading to better patient outcomes. 3D printed anatomic models are especially valuable to physicians when preparing for complex cases or in pediatric cases where the models can be scaled to a size where it is easier for physicians to examine the anatomy. By practicing complex surgeries with 3D printed anatomic models, physicians can determine the best surgical approach for each individual patient, which can lead to better patient outcomes, reduced time in surgery, and cost savings for hospitals and healthcare facilities.
In addition to medical student, patient, and physician education, the 3D printing technology in the Visible Heart Laboratories supports medical device research across a variety of coronary medical device applications. 3D printing technology allows physicians, researchers, and medical device product developers to iterate, create, and test existing and new medical devices in anatomic models that mimic actual anatomy and pathology.
“We believe that widespread use of 3D printed anatomic models in healthcare would translate into better and more cost-effective patient care and shorter time to market for new medical device innovations. This sponsorship allows us to support the world-class education and medical device research happening right here in Minnesota,” said Rich Garrity, President, Americas for Stratasys. “At Stratasys, we recognize the importance of supporting the communities in which we live and work, and we are proud to support the University of Minnesota Visible Heart Laboratories and the work they are doing.”
The Visible Heart Laboratories also are home to the University of Minnesota Atlas of Human Cardiac Anatomy, a collection of more than 800 human hearts that can be recreated with 3D printing technology for education and medical device testing and development.
“We are thankful for those who have donated their heart to the Visible Heart Laboratories, these gifts have allowed the lab to give the gift of life to others,” said Dr. Iaizzo. “Mixed reality education utilizing high resolution 3D printing greatly impacts our abilities to better educate residents, fellows, medical students, biomedical engineers and many others.”
To learn more about the University of Minnesota Visible Heart Laboratories visit www.vhlab.umn.edu. To learn more about Stratasys solutions for healthcare, visit www.stratasys.com/medical.
Stratasys is leading the global shift to additive manufacturing with innovative 3D printing solutions for industries such as aerospace, automotive, consumer products and healthcare. Through smart and connected 3D printers, polymer materials, a software ecosystem, and parts on demand, Stratasys solutions deliver competitive advantages at every stage in the product value chain. The world’s leading organizations turn to Stratasys to transform product design, bring agility to manufacturing and supply chains, and improve patient care.
Contact …..
- USA +800-801-6491
- Europe/Middle East/Africa +49-7229-7772-0
- Asia Pacific +852 3944-8888