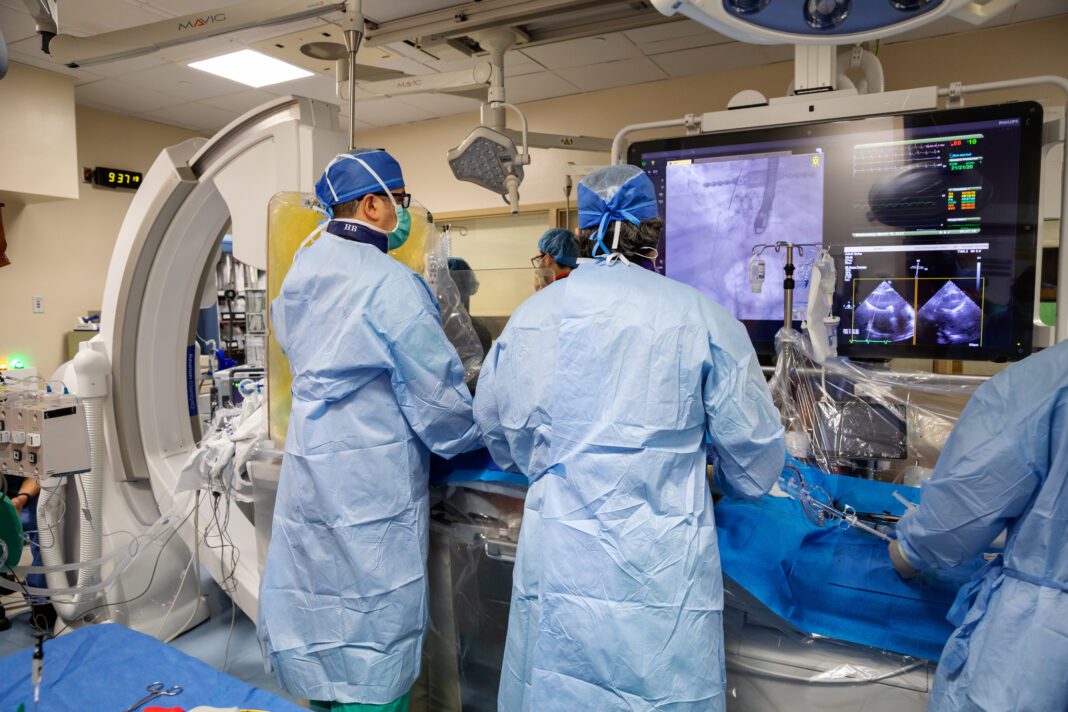
Tampa General Hospital (TGH) and USF Health Morsani College of Medicine Interventional Cardiologists reached multiple milestones this week in being the first in Florida and second in the nation to use a newly developed heart implant designed to replace the tricuspid valve and offer an option to patients who do not meet the criteria for open heart surgery.
The complex population treated by USF Health physicians and Tampa General Hospital also led to one case being the first in the world to implant the TricValve system in a patient with a left ventricular assist device (LVAD).
“The TricValve system represents a new transcatheter technology that could offer patients with symptomatic severe tricuspid regurgitation (TR) and heart failure an option for symptom relief and improved function. It is about a one-hour procedure with fast patient recovery and does not involve fully opening the chest cavity,” said Dr. Hiram Grando Bezerra, professor and section chief of interventional cardiology in the USF Health Morsani College of Medicine and director of the Interventional Cardiology Center of Excellence within the TGH Heart & Vascular Institute.
Tampa General is the second hospital in the country to perform the TricValve procedure and the only one to implant two patients. These procedures were performed on March 15 and 16 by Bezerra and Dr. Fadi Matar, associate professor in the USF Health Morsani College of Medicine and chief of the cardiology department at Tampa General.
“We’re incredibly excited to help these patients, as there is no other option currently available to treat their severe tricuspid regurgitation,” Matar said. “I think this new device has the potential to improve the quality of life for people with this seriously debilitating condition.”
Although transcatheter therapies have become the standard of care procedures over the last decade with aortic and mitral valve therapies, until recently there has been no available transcatheter therapy for the tricuspid valve.
The TricValve Transcatheter Bicaval Valves is a system of two self-expanding biological valves. Specifically, the transcatheter procedure involves going through a patient’s groin and into a main vein to the heart. The two self-expanding valves are deployed at the end of a probe and implanted percutaneously into the inferior and superior vena cava which then replaces the function of the tricuspid valve.
The patients were followed over a period of several months and imaging was analyzed for their potential candidacy to implant the TricValve. Jen Bishop, DNP, APRN lead nurse practitioner for the TGH Interventional Cardiology Center of Excellence commented “We have been able to get to know these patients and their families through this journey and our team is honored to be able to offer them this breakthrough technology. We will continue to follow them throughout their recovery, and it will be exciting to see how the valves will impact their lives and hopefully get them back to doing the things they love.”
TricValve is designated as a breakthrough device by the U.S. Food and Drug Administration (FDA). The FDA’s Breakthrough Devices program gives patients and health care providers timely access to new medical devices. For the procedures performed at Tampa General, the FDA granted a compassionate-use approval to the academic medical center.
“These milestones, especially the first in human application of the TricValve in a patient with a left ventricular assist device, is symbolic of the HVI’s ability to innovate and use – before anyone else –disruptive new technologies to benefit patients,” said Dr. Guilherme Oliveira, professor and chief, division of cardiovascular sciences, USF Health Morsani College of Medicine and executive director, TGH Heart & Vascular Institute. “This represents our commitment to become a destination medical center for cardiovascular care and to continue to define medicine.”
Designed and manufactured by P+F Products + Features headquartered in Vienna, Austria, the TricValve is being studied in clinical trials in the U.S and Europe. Patients are not yet being enrolled in the U.S. trial, which is expected to begin in the near future.