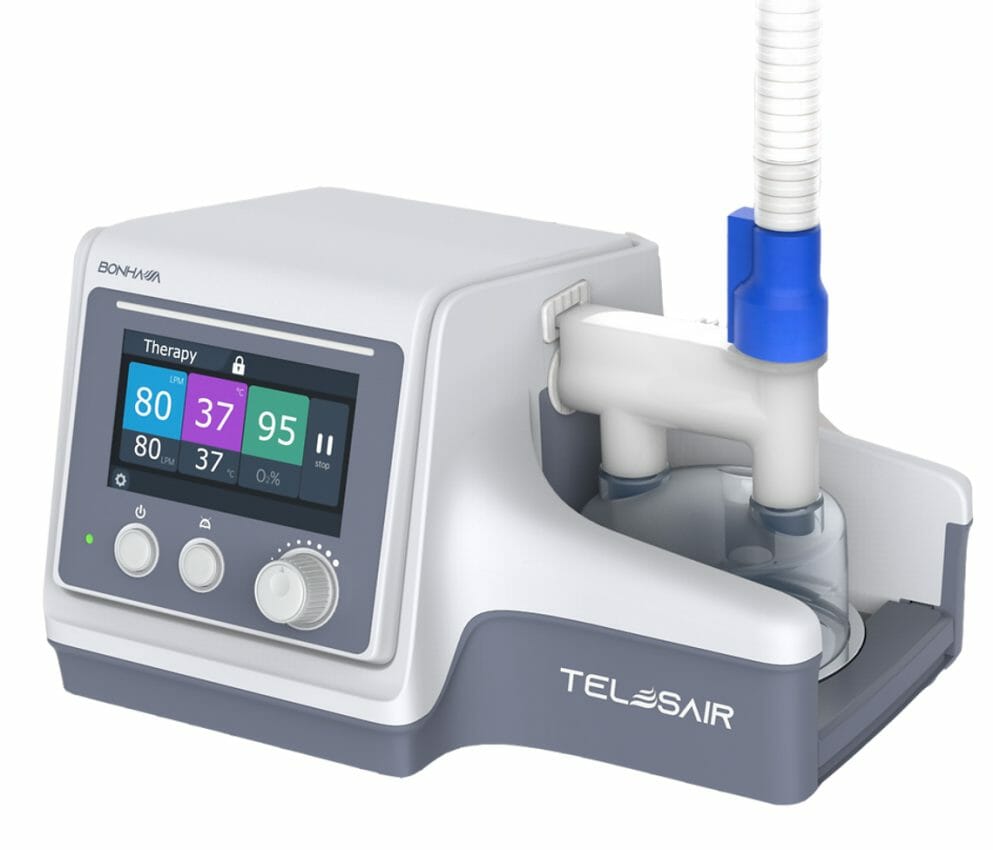
Telesair, Inc., an innovator of next-generation respiratory care, and JPP Care, a leading Thai distributor of respiratory devices, jointly announced today that Telesair’s Bonhawa High Flow Oxygen Therapy (HFOT) system has been approved by Thailand’s Food and Drug Administration (Thai FDA) for use in the treatment of patients with respiratory insufficiency.
The light and compact Bonhawa HFOT device features an extended flow range up to 80 L/pm, a simple disinfection process and an easy-to-use touchscreen, allowing greater therapeutic range, efficient disinfection and the ability to visualize patient settings and data from outside the room.
“Thailand is an important market for us,” said Bryan Liu, CEO of Telesair. “High flow oxygen therapy has become widely accepted at Thai medical centers of excellence and there are many clinicians there that we are eager to work with. Partnering with JPP and its team of knowledgeable specialists also means we will be able to help reach the maximum number of patients while learning about the potential impact of our extended flow range to 80 L/pm.”
“We are excited to represent Telesair’s Bonhawa device here in Thailand because of its enhanced clinical range and the significant potential to innovatively improve the efficiency of patient care and device cleaning,” said Piangpim (Joy) Kirativej, Founder and CEO of JPP Care.
“This latest success was truly a team effort and with our Bonhawa HFOT system now available in Thailand, we’re just getting started,” added Liu. “We’re working toward similar approvals in several other countries, establishing partnerships and studies with multiple institutions, and moving forward with the development of our second-generation home oxygen therapy device. The future for Telesair is very bright!”