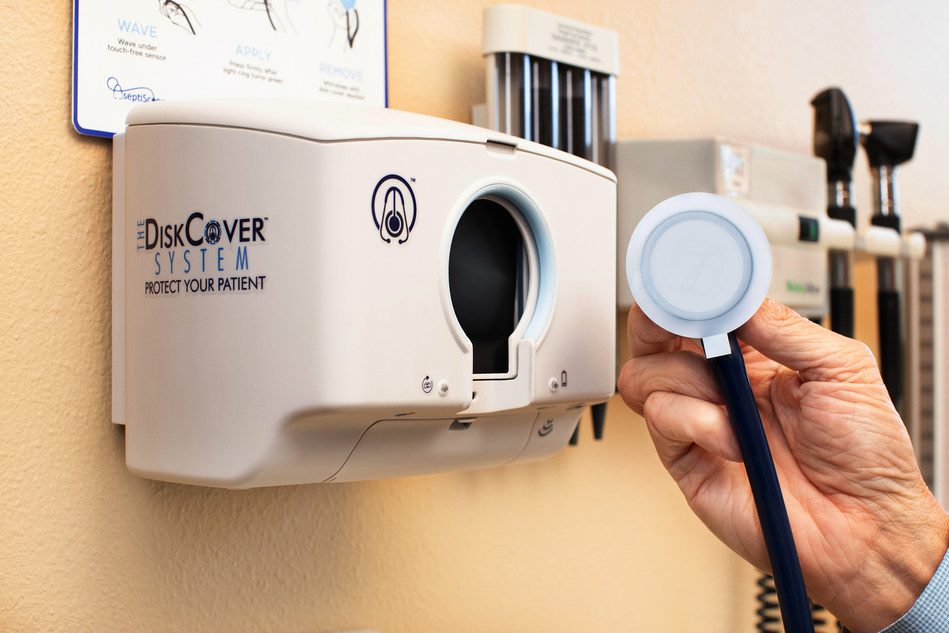
The DiskCover System, the first touch-free stethoscope barrier dispensing system to protect patients from pathogen exposure has been launched by AseptiScope, Inc.
The DiskCover System is the first practical and effective solution to address the longstanding challenge of effective stethoscope hygiene.
“After years of research and development, we are proud to introduce The DiskCover System, the first touch-free stethoscope barrier system to protect patients from pathogen exposure,” said Scott Mader, co-founder and CEO of AseptiScope Inc. “Infection control standards have never been more important, and today’s launch ushers in an infection control solution that is a rapid, highly effective, and affordable method for healthcare providers to protect patients from a well-documented contamination challenge,” he added.
The stethoscope, the most frequently used medical instrument with over 5.5 billion annual auscultations in the US alone, is commonly referred to as the “clinician’s third hand.” It is a ubiquitous, valuable clinical tool and an enduring symbol of the trust between healthcare providers and patients. The stethoscope is also a proven vector of disease transmission, with CDC cleaning guidelines that are incompatible with the intensity of stethoscope use and the high-paced workflow in clinical settings.
Recent observational studies reveal that stethoscopes are seldom cleaned between patients and, even when cleaning does occur, less than 4% of stethoscope diaphragms in these studies met CDC cleanliness requirements. In instances where cleaning techniques such as alcohol swabs are employed in accordance with guidelines, studies have shown that resistant pathogens frequently persist. Moreover, disposable, “single-patient” stethoscope alternatives offer significantly compromised acoustics and fail to address the risk of clinician-to-clinician infections.
“The very low rate of stethoscope hygiene after patient contact represents a current and potentially serious safety threat,” said Dr. Alpesh Amin, Executive Director of Hospital Medicine and Chair of Medicine at the University of California Irvine. In his published research on the subject entitled The Third Hand, Dr. Amin establishes the true scope of the problem. “Transmission of pathogens from patient to patient by stethoscopes could undermine the benefits of hand hygiene programs, as patients are commonly exposed to unclean stethoscopes.”
The DiskCover System is the first and only touch-free stethoscope barrier dispensing system. The System dispenses single-use aseptic disk covers that are proven to protect patients from exposure to harmful pathogens and contaminants on the stethoscope diaphragm without compromising the acoustics critical for accurate diagnoses. The compact DiskCover Dispenser, designed to be mounted in or near hand hygiene stations in the clinical setting, applies individual disk covers instantly, minimizing workflow disruption while encouraging clinician compliance. The DiskCover Dispenser is designed to work with the disposable, cleanroom manufactured Clean Cassette™. Each Clean Cassette houses 420 single-use disk covers and is carefully designed to maintain the aseptic status of each individual disk cover through to the point of care.
With today’s launch, AseptiScope is activating full-scale commercial activities for The DiskCover System and is filling contracted orders with its premier customers; orders that represent a wide spectrum of clinical settings, from leading hospitals to individual physician offices.
“The DiskCover System fills a startling gap in our fundamental efforts to protect patients,” commented Dr. Stuart Kipper of Stuart Kipper MD and Associates in Encinitas California, among the first customers to receive and install The DiskCover System. “As clinicians, we routinely use disposable gloves and gowns to help protect our patients, and ourselves, from pathogens. Now, for the first time, we have a fast and easy system to do the very same for our stethoscopes,” he stated. “I had The DiskCover System professionally installed in our practice and it was up and running in just minutes. In fact, I am already using it and my patients love it,” Kipper added.