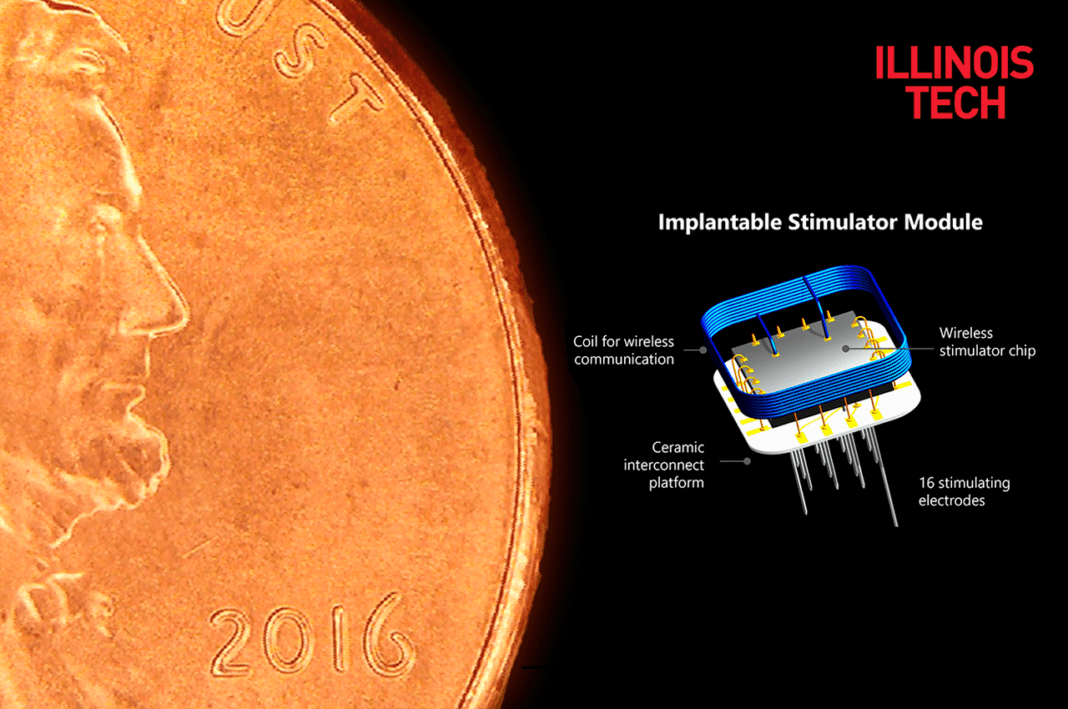
The Intracortical Visual Prosthesis (ICVP), an implant that bypasses the retina and optic nerves to connect directly to the brain’s visual cortex, has been successfully surgically implanted in the ICVP study’s first participant at Rush University Medical Center this week. This surgery is part of a Phase I Feasibility Study of an Intracortical Visual Prosthesis for People With Blindness.
The ICVP system was developed by a multi-institution team led by Philip R. Troyk—executive director of the Pritzker Institute of Biomedical Science and Engineering at Illinois Institute of Technology, professor of biomedical engineering—and represents the culmination of nearly three decades of Illinois Tech research dedicated to ultimately providing artificial sight to those with blindness due to eye disease or trauma.
The Intracortical Visual Prosthesis System is the first intracortical visual implant to use a group of fully implanted miniaturized wireless stimulators to explore whether individuals with blindness can utilize the artificial vision provided by this approach.
This visual prosthesis system allows devices to be permanently implanted, which is a unique advantage that provides researchers ample time to explore how the device can effectively work, and for the recipient to learn how the device can be useful.
During the preclinical phase, the Illinois Tech team worked with Rush University Medical Center neurosurgeons to develop and refine surgical procedures, culminating in this week’s successful implantation of 25 stimulators with a total of 400 electrodes in an individual with blindness. The clinical phase is aimed at testing whether this prosthesis will provide study participants with an improved ability to navigate and perform basic, visually guided tasks. Testing will commence at The Chicago Lighthouse after a 4-6-week recovery period.
“This is an incredibly exciting moment, not just for the field of biomedical engineering, but more importantly for people with blindness and their loved ones around the world,” says Troyk.
Since many individuals affected by total blindness do not have intact retina or optic nerves but retain the visual cortex—the area of the brain that allows people to see—an intracortical visual prosthesis may be the only possible advanced visual sensory aid from which they can benefit.
While the brain works as a powerful processing system and receives millions of nerve signals from the eyes, if the eyes are no longer able to communicate with the brain, Troyk says that researchers can “intervene by bypassing the eye and optic nerve and going directly to the area of the brain called the visual cortex.”
“This operation represents a critical step in the decades of research by our whole ICVP team in our efforts to bring sight to blind patients,” says Dr Richard Byrne, the neurosurgeon at Rush University Medical Center who performed the surgery.
Illinois Tech is collaborating with Rush University Medical Center, The Chicago Lighthouse; the Wilmer Eye Institute at Johns Hopkins; the University of Texas at Dallas; Microprobes for Life Science; Sigenics, Inc.; and The University of Chicago on the initiative, with Troyk serving as the principal investigator.
“For people who are completely blind, gaining even a little bit of light perception can make a huge difference,” said Janet P. Szlyk, President and CEO of The Chicago Lighthouse. “The findings from this research will help pave the way for other groundbreaking advancements in blindness research and vision restoration.”
Funding for this study was provided by the National Institutes of Health’s Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, the Department of Defense and from private donors.