November 3, 2020
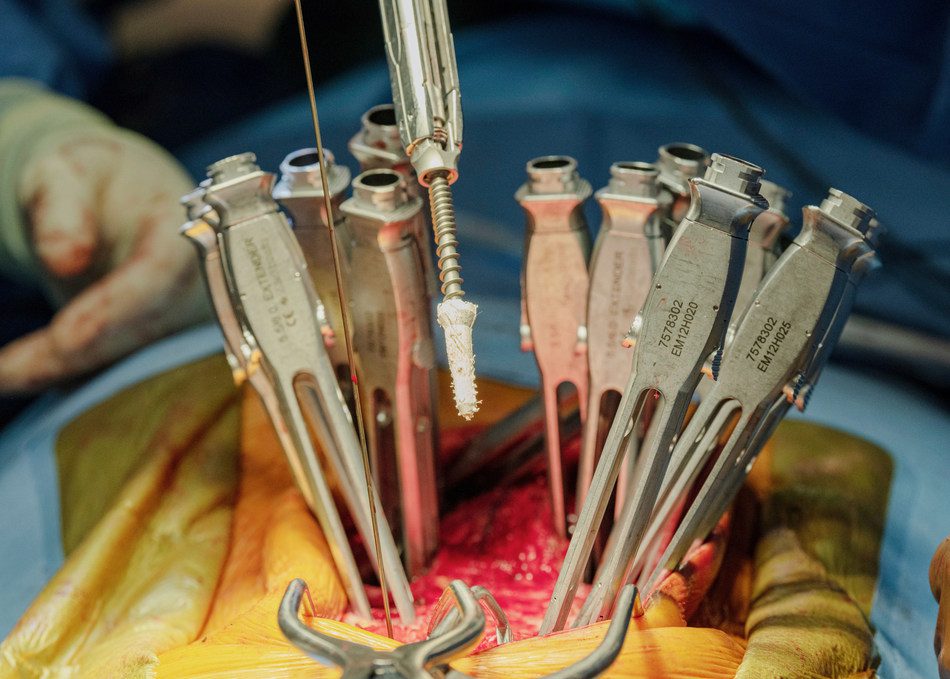
TheraFuze DBF Fiber Anchor: TheraCell today announced they completed the first three spine surgical cases using the world’s first-ever demineralized bone fiber screw anchor.
TheraFuze DBF Fiber Anchor is a unique product and acts much like a drywall anchor that provides immediate improvement in screw fixation in primary and revision cases.
One of the cases was performed by Dr. Neel Anand, MD, Mch. Orth. (Liverpool), FAAOS, Professor of Orthopaedic Surgery and Director, Spine Trauma, Cedars Sinai Medical Center, and Minimally Invasive Spine Surgery, DOCS Surgical Hospital, Beverly Hills. The case was a T-11 to Ilium adult MIS deformity revision with bilateral fractured rods at the levels of pseudoarthrosis. Dr. Anand used Fiber Anchors to secure screws at three levels. Further, he employed two TheraFuze DBF® Fiber Wraps™ with 10cc of TheraFuze DBF FiberForm™+ for a two-level fusion.
“I was extremely interested to use the Fiber Anchor in this revision case because of the tremendous potential for improved fixation at the proximal and distal ends of the construct. The improved fixation was tactilely obvious on screw insertion and the entire procedure was very simple and fit into my usual process of pedicle preparation and screw placement with ease. I also was impressed with the Fiber Wrap. We rolled pieces of Fiber Wrap with some autologous bone graft and TheraFuze DBF containing mineralized cortical chips to make four “Burritos”. It made graft placement very easy and controlled. The Fiber Anchor and the Fiber Wrap are game-changers. I have additional surgeries scheduled and will continue to use the products.”
The second case was completed with Dr. Anis Mekhail, MD Orthopedic Spine Surgeon, Assistant Clinical Professor of Orthopedics at University of Illinois in Chicago. Dr. Mekhail confirmed the concept of utilizing the fiber anchor for pedicle screw augmentation. He plans to actively use the technology rather than PMMA on appropriate patients in the future.
The third case was completed By Dr. Frank Phillips, The Ronald DeWald, Endowed Professor of Spinal Deformities, Director of the Division of Spine Surgery and Section Head of Minimally Invasive Spine Surgery at Rush University Medical Center. Dr. Phillips used Fiber Anchors in a revision deformity surgery to augment S2AI (sacro-iliac) screws at the caudal end of a deformity multi-level instrumented fusion. Dr. Phillips stated, “the increased purchase of the S2AI screws with the fiber anchors was readily discernable during the surgery and resulted in excellent screw fixation. This technology adds immense value in longer deformity constructs where the loss of hardware fixation and resultant failure of fusion is a significant concern.”
Curt Cooper, TheraCell’s Chief Business Development Officer said, “The TheraFuze DBF Fiber Anchor offers a cost-effective solution for revision cases or where the surgeon “feels” the bone quality is compromised as it provides significant improved initial in vivo fixation. In our pre-clinical studies, the Fiber Anchor demonstrated that it promotes new bone formation in apposition to the screw with interdigitation of remodeled bone into the threads surrounded by native bone. Proud to develop a highly differentiated clinical solution, we believe the Fiber Anchor provides surgeons a novel, safe alternative to Polymethyl Methacrylate (PMMA).”
The TheraFuze DBF Fiber Anchor is made from the company’s proprietary demineralized cortical bone fibers. Testing demonstrated it more than doubles the pullout force versus screws without the Fiber Anchor. TheraFuze DBF Fiber Anchors are sized to be used with 5.5-6.0mm, 6.5-7.0mm, 7.5-8.0mm, and 8.5-9.0mm screws. The TheraFuze DBF Fiber Wrap contains demineralized bone fibers formed into a 1mm thick sheet with TheraCell’s FiberLok™ technology. The lyophilized sheet may be rehydrated with saline, the patient’s blood, or bone marrow aspirate creating a flexible sheet that can be manipulated by the surgeon.
It can be rolled up with autograft for graft containment and laid in the posterolateral gutters for PLF. The FiberLok process maintains the forms integrity when hydrated. TheraFuze DBF FiberForm+ is a moldable demineralized excipient free putty designed for use in orthopedic and spine surgery that also includes mineralized cortical chips. Cortical chips provide additional compression resistance and radiodensity for visualization. All of the TheraFuze DBF products are 100% cortical bone fiber products that are osteoconductive with osteoinductive potential to stimulate long term bone formation.