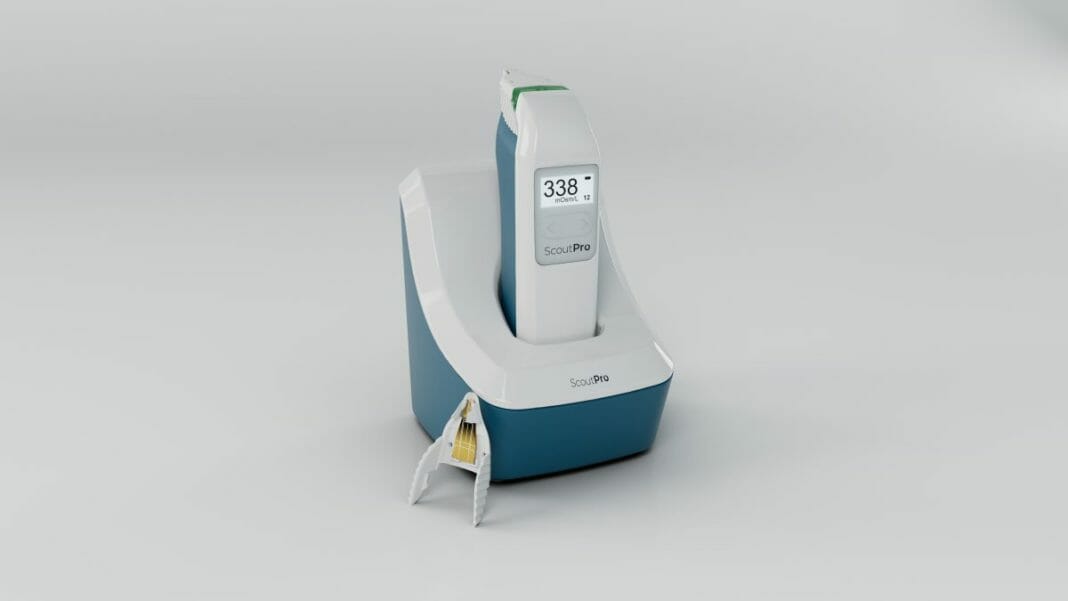
Trukera™ Medical last week announced a corporate rebranding to reflect the company’s expanded vision and strategic growth plans targeting broader unmet needs in corneal health. Today, Trukera Medical unveiled the first product under the new company name, the ScoutPro™ Osmolarity System. Designed to help advance corneal health decisions for today’s busy eye care practices, ScoutPro is the first and only portable osmometer in the U.S.
“The ScoutPro Osmolarity System is an exciting technology developed around the evolving needs of today’s eye care professionals,” said Adam Szaronos, Trukera Medical CEO. “Premium cataract and refractive practices are now the fastest growing adopters of osmolarity testing, given the prevalence of hyperosmolarity in their patient demographics and the risk it poses to compromising corneal cell health and driving refractive instability. More than ever, these practices require patient care solutions that can seamlessly adapt into established operational models, not the other way around, to deliver a premium patient experience, efficiency of care, and minimize staff time and disruptions. We’re proud to bring a solution to market addressing these needs, and to help more practices objectively identify and manage such a critical component to patient’s corneal health and refractive outcomes.”
The ScoutPro Osmolarity System brings together nanoliter volume sample collection and analysis in a single portable device. Users can now bring laboratory testing to patients anywhere in the practice to quickly test and provide objective results in the palm of their hand. The system is built upon the company’s established accuracy trusted in over 24 million tests globally to date, with an industry-leading precision.
“Toxic hyperosmolarity damages sensitive corneal nerves and may cause refractive instability. As cornea and cataract/refractive specialists, we are cognizant of the importance of this to our surgical outcomes and the overall health of our patients’ eyes. ScoutPro gives us the ability to precisely determine osmolarity quickly and easily, without disrupting patient flow,” said Dr. Lisa Nijm, Chief Medical Advisor for Ophthalmology at Trukera Medical and Corneal Surgeon at Warrenville Eye Care and LASIK Center in Illinois.
The ScoutPro Osmolarity System is now available for pre-order in the U.S. and will be commercially launched at the upcoming World Cornea Congress and the American Academy of Ophthalmology later this month. Visit www.trukera.com to learn more about the ScoutPro Osmolarity System from Trukera Medical.