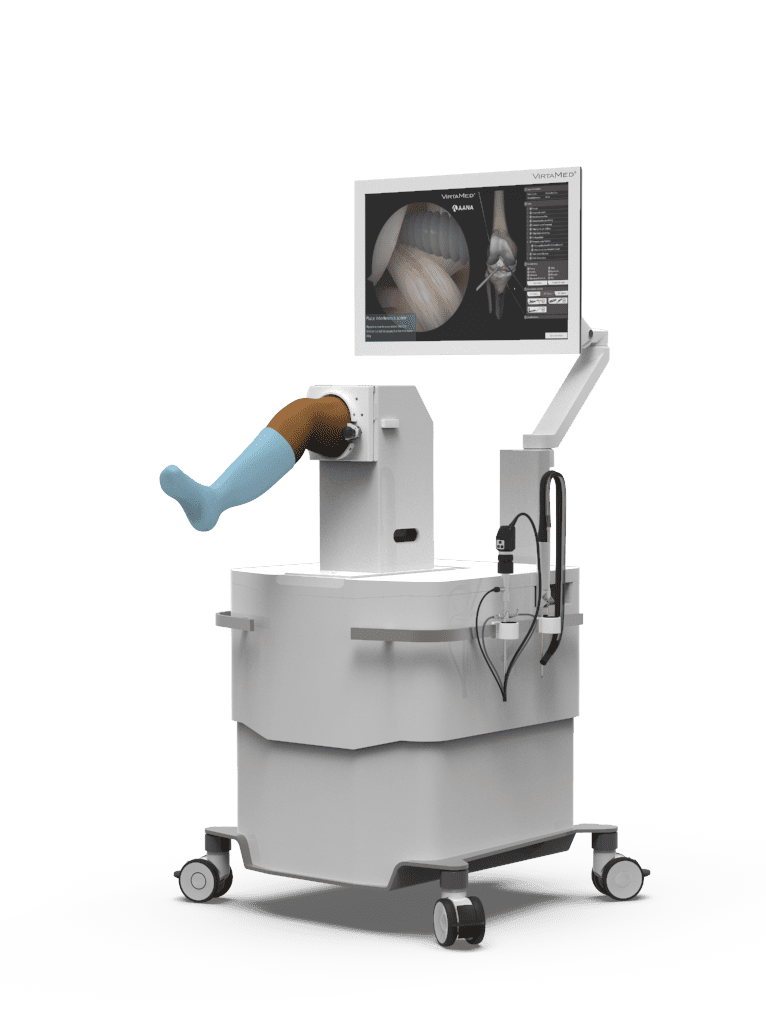
VirtaMed, the world leader in medical simulation training, has announced the release of enhanced simulation training modules for arthroscopic knee surgeries. In a first for VirtaMed, these latest training modules will now be available on simulators with two different skin tones.
“It is important to learn medical skills in a realistic environment, and that means training on simulators that reflect the diversity of both patients and trainee physicians,” said Dr. Raimundo Sierra, founder and CEO of VirtaMed. “VirtaMed is committed to encouraging diversity and inclusion in the education of medical skills, and we believe these values should also be reflected in the simulator hardware. This is just one step in the right direction toward more equitable healthcare education and delivery.”
A 2005 report released by the Institute of Medicine documented that “racial and ethnic minorities receive lower-quality health care than white people — even when insurance status, income, age and severity of conditions are comparable.” While not referring specifically to surgical outcomes, the report and similar studies have indicated that people of color are less likely to receive effective treatments, due in part to implicit bias from healthcare providers.
In developing the new simulators, VirtaMed collaborated with knee experts Dr. Jacqueline Brady, Dr. Cassandra Lee, and Dr. Patrick Joyner from the AANA Knee Taskforce. VirtaMed and AANA knee experts identified high-priority new cases and features with the most potential impact for training the next generation of surgeons. The VirtaMed ArthroS™ is unique in simulation training as trainees are able to palpate and distract a physical model while at the same time performing a virtual surgery.
The new training options are the most recent in a series of updates stemming from a collaborative agreement between VirtaMed and the AANA to advance and improve arthroscopy surgery skill training and develop standards for proficiency-based training curricula. Since 2018, AANA experts have collaborated with VirtaMed to provide expert input on cases and courses for training on the fundamentals of arthroscopy (FAST), and the knee, shoulder and hip joints.
The new model, as well as additional software upgrades, will be unveiled at this year’s American Academy of Orthopaedic Surgeons (AAOS) conference held in Chicago, March 22-26. 2022. Live demonstrations will be held at booth 1421 and can be reserved at virtamed.com.