Vivalink, a leading provider of digital healthcare solutions, today announced the results of a company survey focused on clinicians’ use of Holter monitors versus mobile cardiac telemetry (MCT).
The survey results indicate 68 percent of respondents said using MCT over Holter monitors provides better detection rates and more accurate diagnoses for patients.
Vivalink notes the survey asked 100 U.S. clinicians, including cardiologists, electrophysiologists, nurse practitioners and physician assistants if the Holter monitor, invented in the 1950s, still meets the needs of patients and providers. The survey revealed some comparative advantages and disadvantages of Holter monitors versus MCT, including the following findings:
-
The primary advantage of Holter monitors (52 percent of respondents) was ease-of-use, whereas better detection and diagnosis was cited as the main advantage for MCT (68 percent of respondents)
-
Forty-eight percent of respondents said that the biggest disadvantage of using Holter monitors was that they are bulky for patients compared to 50 percent of the respondents noting complexity as the main disadvantage for MCT
-
Nearly 20 percent of respondents said that the delay in getting results was the second-biggest disadvantage of using Holter monitors versus 25 percent saying more training required is the second-biggest disadvantage of MCT
In addition to comparing Holter and MCT features, respondents also addressed how much they might use each device in the future. About 63 percent of respondents said they expect a slightly to significantly higher use of Holter monitors in 2022, whereas 58 percent of respondents indicated the same for MCT. While MCT use is expected to grow this year, Holter monitors are not being phased out in the near future. Finally, 45 percent of respondents said they prescribe MCT (directly or indirectly) at about the same rates as Holter monitors, and 50 percent of respondents said better awareness among clinicians is the main factor in increasing the use of MCT.
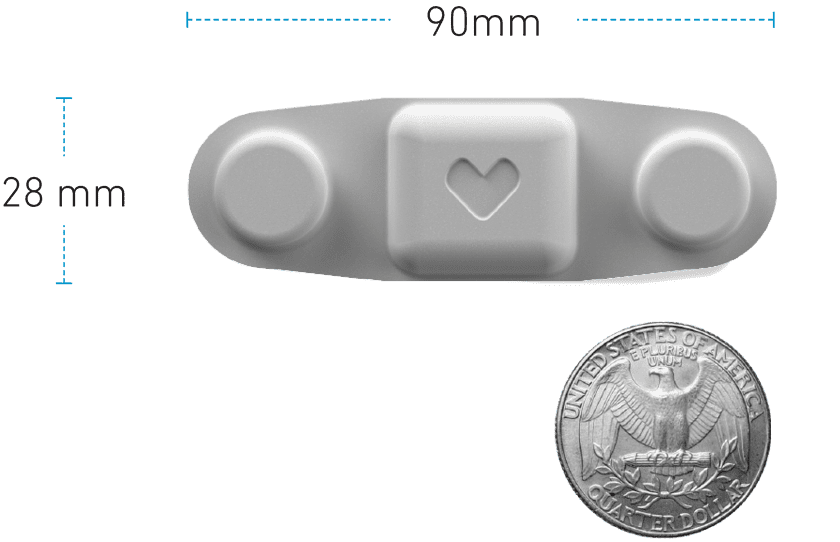
As an alternative to bulky wired Holter monitors, Vivalink’s reusable and rechargeable
Cardiac ECG Patch is the world’s smallest medical-grade ECG monitor of its kind, with the ability to serve both Holter and MCT based cardiac monitoring. This powerful cardiac patch has been used in multiple studies assessing atrial fibrillation, coronary artery disease, stress and depression, and more.
“The feedback from this survey has some interesting insights into the advantages and disadvantages of Holter and MCT. While it is expected that Holter monitors will eventually wane, the respondent data suggests that it’s not going away any time soon due to its ease-of-use and familiarity by clinicians,” said Jiang Li, founder and CEO of Vivalink. “However, MCT usage is continuing to grow and has new benefits such as better detection with faster results which can lead to a faster and more efficient medical diagnosis.”
Vivalink has been serving more than 100 healthcare application partners and customers in 32 countries with a Biometrics Data Platform as a Service business model. Some of Vivalink’s customers, such as IDTF’s, are using its innovative reusable ECG patch and Data Platform for MCT and Holter services. Vivalink’s solution has also been used in supporting research institutions such as UCSF, Emory University, and Stanford for cardiovascular and cardiac related research activities.