South Korean artificial intelligence (AI) developer, VUNO inc. announced that the Korean Ministry of Food and Drug Safety (KFDA) has designated the company’s artificial intelligence-based electrocardiogram (ECG) analysis software VUNO Med®-DeepECG™ as the ministry’s 16th Breakthrough Device. Using deep learning, the software analyzes ECG data to zero in on heart failure, myocardial infarction and arrhythmia.
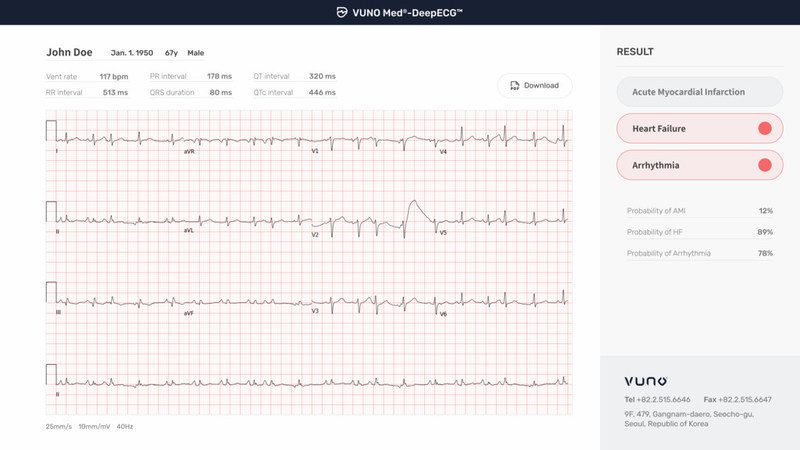
Although healthcare authorities such as the U.S. Preventive Services Task Force have pointed out the limitations of ECG tests on identifying cardiovascular diseases, the VUNO Med®-DeepECG™ can pinpoint even minute differences in ECG data that escape visual analysis by learning the ECG data of patients suffering from heart failure, myocardial infarction and other major heart-related disorders. Through this learning process, the device provides additional information on heart failure undetected in previous ECGs to identify a myocardial infarction even if no major deflections in the ECG wave patterns can be seen.
VUNO Med®-DeepECG™, once implemented in clinical practice, could help reduce mortality rates from major heart diseases and improve patient quality of life through early detection of symptomless patients through ECG testing and timely treatment.
VUNO Chairman Lee Yeha said, “VUNO will continue to deliver the value of AI to even more beneficiaries through a wide range of biosignal initiatives, including the use of ECG data with its significant potential for providing clues to serious heart-related diseases.”
VUNO is the only Korean company to be triple-designated for Breakthrough Devices, with the VUNO Med®-Fundus AI™ selected as Korea’s first Breakthrough Device and the VUNO Med®-DeepCARS™ receiving the sixth designation.


