Widex Inc. has published a survey that highlights the importance of sound quality in hearing devices and validates the company’s philosophy that natural sound is the most important feature because it supports social participation in everyday life.
“While hearing aids continue to evolve to include new features, we believe that quality of sound is and will always be central. Natural sound enables a hearing aid wearer to react to external stimuli – the sounds of the environment, the voices of family and friends, and more.
This helps keep their minds active, and in turn, preserve brain function,” said Laura Winther Balling, Evidence and Research Specialist at Widex. “This new report serves to further emphasize the role that a hearing aid’s natural sound quality plays in this critical daily social participation.”
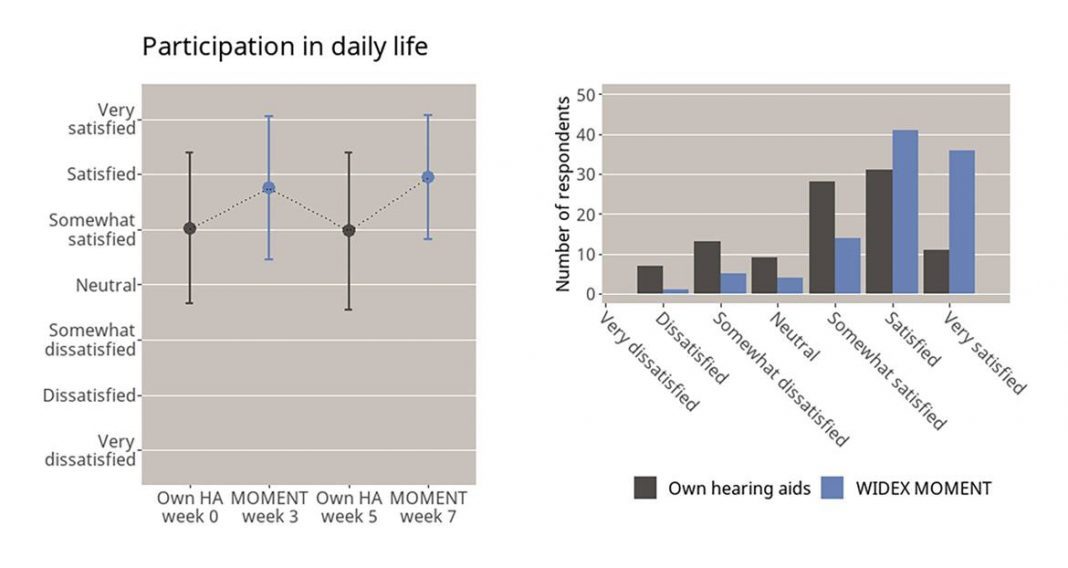
The international survey included 101 experienced hearing aid users in seven countries including the United States, Canada, China, Germany, France, Portugal, and the United Kingdom. Each participant was fitted with WIDEX MOMENT hearing aids and asked to rate their satisfaction with them in comparison to their own hearing aids from various brands.Their responses suggest that high-quality sound better enabled their ability to participate in real-life moments and encounters. When asked how satisfied they are with their ability to participate effortlessly in everyday life, only 69% indicated that they can with their own hearing aids, while a whopping 90% indicated effortless everyday participation with the WIDEX MOMENT.
Overall, participants were reasonably satisfied with their own hearing aids at the beginning of the survey period, but after trying the WIDEX MOMENT, ratings of their own hearing aids significantly lowered. Their responses indicated that natural sound quality – which Widex is known for – is a key feature for overall hearing aid satisfaction.
“The fundamental purpose of hearing aids is to restore the ability to experience life, and this report showed how critical sound quality is to achieving that goal,” Balling emphasized. “Unfortunately, according to recent studies, even slight hearing loss can result in social isolation, depression, anxiety and cognitive decline. Meanwhile, social factors have been shown to positively impact health outcomes. Being able to participate socially has serious implications for the health of the aging population – which is why a hearing aid’s ability to provide natural sound is so important.”
Widex is committed to providing the best sound experience possible. WIDEX MOMENT hearing aids use proprietary technologies to create a more perfect, natural sound that’s uniquely and automatically tailored to each wearer’s listening preferences and profile. Its PureSound™ ZeroDelay™ technology all but eliminates processing latency, resulting in a more natural sound without the “tinny” distortions associated with other hearing aids. Plus, its SoundSense Learn technology leverages artificial intelligence to create a more personalized sound experience adjusted to the wearer’s listening environment.
To read the entire survey, visit The Hearing Review’s article here.