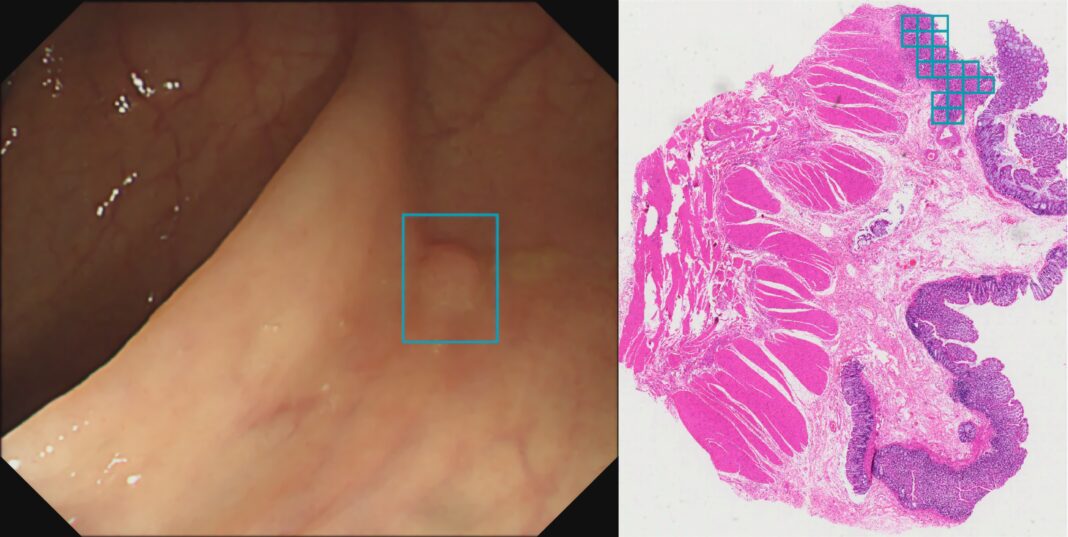
Wision AI Ltd, a startup in the field of artificial intelligence assisted diagnostics for optical medical imaging, today announced the expansion of its product portfolio with recent U.S. Food & Drug Administration (FDA) 510(k) Clearance for EndoScreener, its AI-assisted polyp detection software during colonoscopy, and an FDA presubmission in queue for an upcoming histopathology AI software that assists in the localization of high-grade dysplasia (HGD) in whole slide imaging (WSI).
According to the American Cancer Society, colorectal cancer (CRC) is the second most common cancer in men and women combined and is expected to cause over 50,000 deaths in 2022.i Detection and removal of adenomatous polyps at early diagnosis and regular colonoscopy screening are the most effective ways to reduce the incidence and mortality of CRC. Following the data publication surrounding the first external independent randomized controlled trial (RCT) of AI in the United States, in November 2021, the FDA granted 510(k) Clearance for EndoScreener, Wision A.I.’s AI-assisted polyp detection software. The software-only medical device increased detected adenoma by 32.2% and reduced adenoma miss rate by 35.6% in a multi-center randomized controlled trial conducted by four U.S. leading academic medical centers.ii
The Centers for Disease Control (CDC) estimates that by 2024, there should be approximately 11 to 13 million annual colonoscopies needed to screen the eligible patient population.iii The emergence of AI, as well as other endoscopic technologies, is proven to considerably increase polyp detection and removals per colonoscopy. Wision A.I.’s new histopathological AI product is used to localize HGD, the cancerous characteristics, in histologic whole slide imaging (WSI) for colorectal specimens. Through this technique, a precise histological diagnosis is used to determine a patient’s risk and surveillance interval, identifying high-risk signs that lead to interval CRC. The incorporation of AI improves efficiency during histopathological diagnosis by saving the pathologist time to determine the correct diagnosis for each patient.
“Obtaining 510(k) Clearance from the FDA for EndoScreener and initiating a U.S. based investigational validation of the new tool for histopathological diagnosis demonstrates how our broadened, enriched AI portfolio can help to reduce colorectal cancer and improve patient outcomes,” stated JingJia Liu, co-founder and Chief Executive Officer of Wision A.I. “This announcement comes at a pivotal time during Colorectal Cancer Awareness Month, where we are working towards optimizing CRC detection through new, efficient, and precise methods. We believe that AI polyp detection in colonoscopy and AI HGD localization in histological diagnosis will greatly synergize and contribute to CRC screening and prevention.”
References
i Colorectal cancer statistics: How common is colorectal cancer? American Cancer Society. (n.d.). Retrieved March 11, 2022, from https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
ii Glissen Brown JR, Mansour NM, Wang P, et al. Deep Learning Computer-aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-center Randomized Tandem Colonoscopy Study (CADeT-CS Trial) [published online ahead of print, 2021 Sep 14]. Clin Gastroenterol Hepatol. 2021;S1542-3565(21)00973-3. doi:10.1016/j.cgh.2021.09.009
iii Joseph DA, Meester RG, Zauber AG, et al. Colorectal cancer screening: Estimated future colonoscopy need and current volume and capacity [published correction appears in Cancer. 2017 Oct 1;123(19):3857]. Cancer. 2016;122(16):2479-2486. doi:10.1002/cncr.30070