xMEMS Labs today introduced Montara Pro, the world’s first monolithic MEMS µspeaker with integrated DynamicVent enabling smart TWS earbuds and hearing aids that create best-of-both-worlds user experiences combining the benefits of closed-fit (occluded) and open-fit earbuds. xMEMS is providing first demonstrations of Montara Pro this week at the Consumer Electronics Show in Las Vegas at Venetian Suite 29-325.
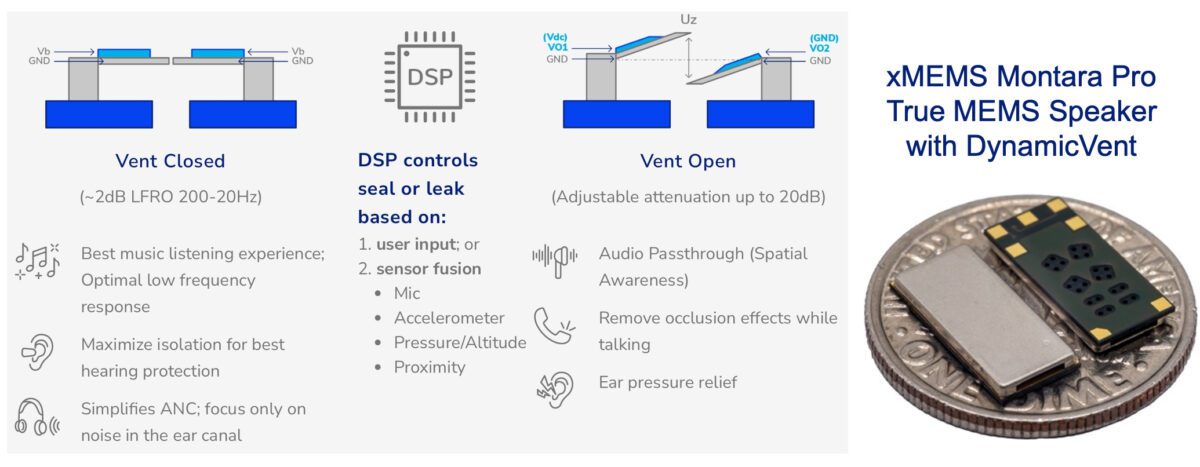
Montara Pro’s patented DynamicVent technology can be intelligently opened or closed by the earbud system DSP based on ambient noise levels detected by microphones or the listener’s activity from motion sensors. With the vent closed, Montara Pro creates a listening environment with the best passive isolation for music and media consumption or for improved focus in the presence of background noise. With the vent open, Montara Pro enables improved spatial awareness, increased listening comfort, and reduced occlusion effects, such as the perception of the user’s own voice as too loud, “boomy,” or “hollow.” Equally important, DynamicVent eliminates the need for traditional static vents that create a persistent low frequency roll-off that impacts music and media quality and also impacts consumers with low-frequency hearing loss. The DynamicVent can also eliminate the need for oversized speakers in earbuds that are commonly used to compensate for low frequency loss caused by persistent, static vents.
“As part of our mission to ‘Reinvent Sound’ we are extending the capabilities of what µspeakers can do in small form-factor systems,” said Dr. Chiung Lo, xMEMS Co-Founder and VP of Design. “Achieving the highest quality sound reproduction remains job-one, but now with DynamicVent technology we enable system designers to balance the benefits of open and closed-fit earbud architectures that lead the way to more intelligent and higher-performance solutions for consumers in a variety of listening environments.”
“Venting is critically important to TWS earbuds and hearing aids. In the past, trade-offs had to be made when selecting either an open-fit/vented or closed-fit/occluded implementation and neither choice provides the best listening performance in all environments and life situations,” said Dr. Abram Bailey, AuD. and CEO of Hearing Tracker, Inc. “The emergence of active venting technologies, like DynamicVent from xMEMS, is a windfall for both consumers and hearing care professionals, eliminating trade-offs and enabling consumer audio products and listening performance that can adapt to any environment.”
Montara Pro’s µspeaker delivers a flat frequency response achieving 115dB SPL up to 1kHz and provides up to 18dB of gain above 1kHz for improved voice and instrument clarity. Montara Pro is a monolithic, single-die architecture implementing both the speaker and the DynamicVent in silicon resulting in unmatched part-to-part frequency response consistency and reducing speaker matching or calibration time at manufacturing. This innovative transduction mechanism has also produced the world’s fastest and most precise µspeakers, eliminating spring and suspension recovery of coil speakers which improves audio quality and sound field reproduction.
Montara Pro’s DynamicVent implements a differential design that eliminates open/close noise. The large vent opening creates relief equivalent to a 1.5mm2 hole and offers 20dB of attenuation from 500Hz to 20Hz. Unlike other non-MEMS approaches to active venting, the DynamicVent achieves an IP58 rating providing better resilience to particulate and moisture ingress, improving the longevity of the end-product.
Availability
Montara Pro samples and evaluation kits are available today for select customers, with mass production expected in Q3 2022. Montara Pro is available in a side-firing (5.15 x 1.15 x 10.8mm) LGA package. Montara Pro is paired with the xMEMS Aptos Class-H audio amplifier (1.92 x 1.92 x 0.6mm WLCSP).


