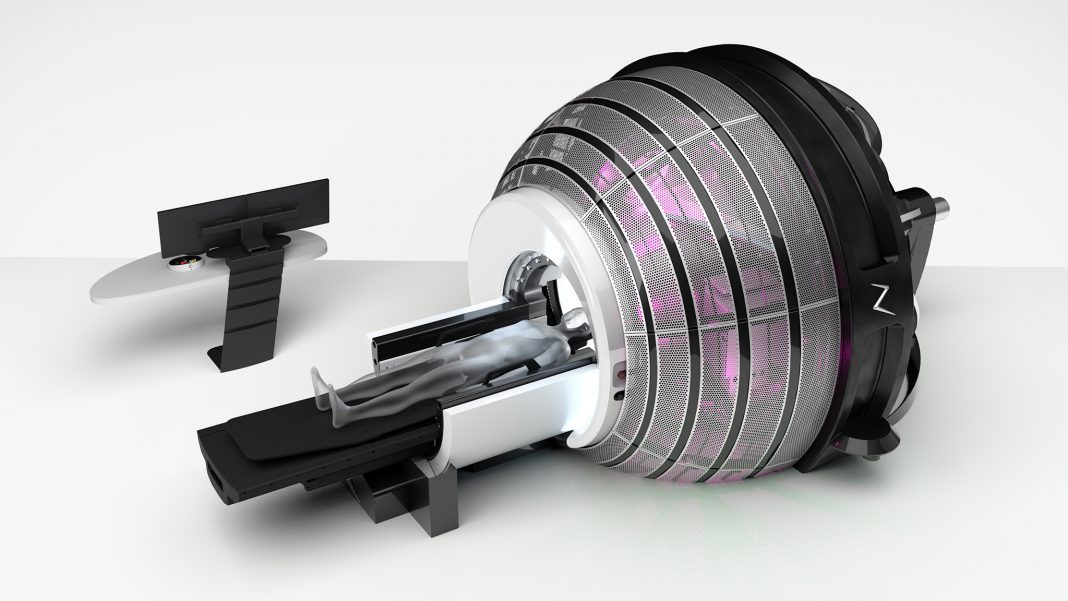
ZAP Surgical Systems, Inc. today announced the imminent installation of its advanced ZAP-X® Gyroscopic Radiosurgery® platform at the Swiss Neuro Radiosurgery Center (SNRC), an organization within the Swiss Clinical Neuroscience Institute (SCNSI) in Zurich, Switzerland.
Stereotactic radiosurgery (SRS) is a well-studied and effective treatment for many brain cancers including primary and metastatic brain tumors, as well as select intracranial functional and vascular disorders. Considered an alternative to surgery for these indications, SRS is a non-invasive outpatient procedure that often provides equivalent to superior outcomes, yet no surgical incision, and little to no recovery period.
The ZAP-X system is recognized for using gyroscopic motion to direct radiosurgical beams from a multitude of unique angles which precisely concentrate radiation to the tumor target, resulting in significantly less healthy brain tissue exposure than those of multi-purpose radiation delivery systems. This unique approach supports the clinical objective of protecting healthy brain tissue and neuro-cognitive function.
ZAP-X is also the first and only dedicated radiosurgery system to no longer require Cobalt-60 radioactive sources, thus eliminating the significant costs to license, secure and regularly replace live radioactive isotopes.
“The local core-team consisting of neurosurgeon Christoph Weber, MD, radiation oncologist Cristina Picardi, MD, and myself are looking forward to putting Switzerland’s first ZAP-X into operation,” said Andreas Mack, PHD, founder and chief executive officer of SNRC.
“With an interdisciplinary focus on neuro-radiosurgical treatments, the project is a cooperative effort including the Klinik Hirslanden Institute for Radiotherapy, the Center for Endoscopic and Minimally Invasive Neurosurgery, namely Robert Reisch, MD, and Nikolai Hopf, MD, and the Center for Micro Neurosurgery, namely Ralf Kockro, MD,” added Mack. “Additionally, SNRC is scientifically advised by the clinical team at the University Hospital of Basel, Department of Neurosurgery, namely Luigi Mariani, MD, Raphael Guzman, MD, and Ethan Taub, MD.”
“ZAP Surgical is excited to partner with SNRC to bring world-class radiosurgery to Zurich and the surrounding communities,” said Hakan Baraner, ZAP Surgical’s Senior Vice President for Europe, Middle East, and Africa. “We believe SNRC’s ZAP-X program will serve as an example to many other medical centers, both locally and globally.”
SNRC estimates first patient treatments with ZAP-X will commence in the summer of 2021.