December 29, 2020
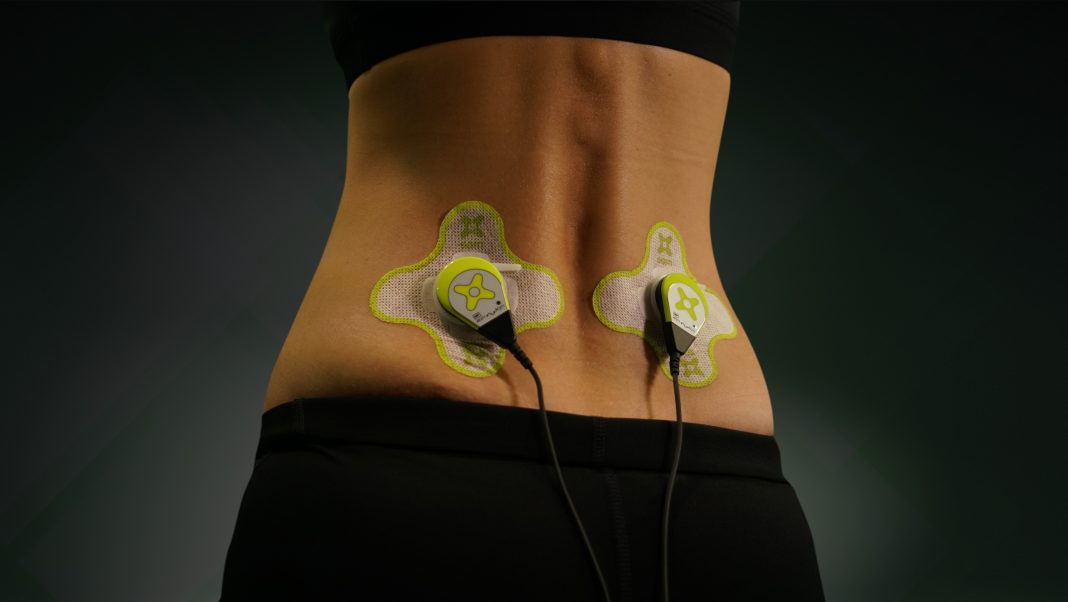
ZetrOZ Systems, developers of the Sustained Acoustic Medicine (SAM) wearable ultrasound, an FDA-cleared bio regenerative medical device, is the global leader in development of SAM technologies.
With its reapproval for expanded use in 2020, the use of SAM ultrasound technology in both professional and at-home applications is expected to increase market growth significantly over the next several years.
Video here.
“The benefits of SAM are growing clearer by the day as more research-based evidence becomes available supporting SAM’s ability to provide healing relief without surgery,” according to Dr. George Lewis, Founder and CEO of ZetrOZ. “More professional athletic organizations and sports medicine doctors are taking notice, and we hope that more people will come to learn about the deep healing relief that SAM technology can provide.”
SAM technology is backed by tens of millions of dollars from the National Institutes of Health (NIH) and Department of Defense (DoD) for its ability to treat pain without surgery or opioids. The use of SAM is being integrated into clinical training with plans to expand into commercial insurance markets to treat patients; over 300,000 patients have been treated across government agencies, including Veterans Affairs, DoD, and Department of Labor (DoL), and the private sector.
Multiple studies are always underway to increase the knowledge of SAM in sports medicine, pain management, and rheumatology. In addition to research on SAM products, ZetrOZ is developing a pipeline of additional bioelectronic devices to improve healthcare and society. ZetrOZ has a vision to help 100 Million patients suffering from chronic pain and soft-tissue injuries.