oVio’s New AI Enhanced, Fully Dimensional Imaging Technology May Be a Game Changer for Healthcare & Security Sectors
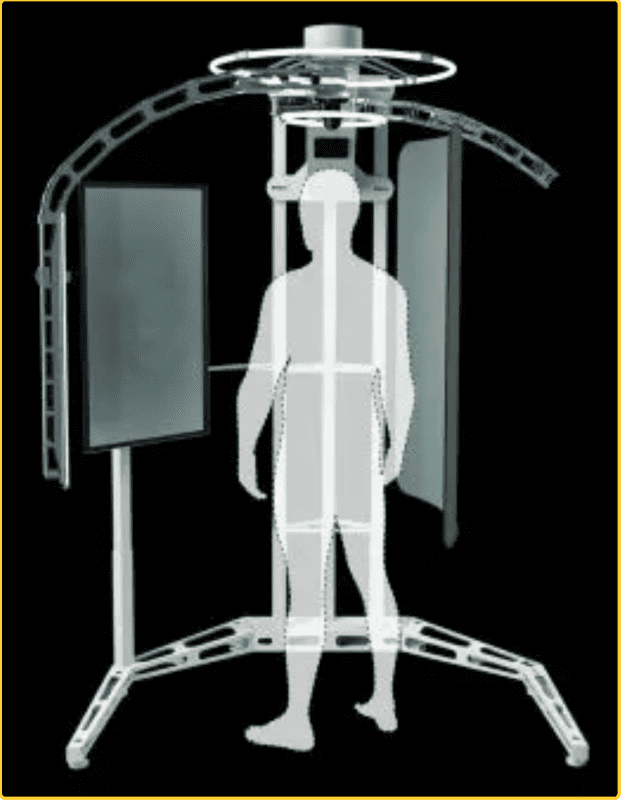
oVio’s imaging technology has been heavily used and adopted by medical practitioners in cosmetic and elective surgery specialties, allowing for patients to have 360-degree views of everything from before-and-afters to prospective post-op results. Their existing products are well-established as an invaluable resource to doctors providing cosmetic surgery, skincare, hair replacement treatment, and other services to their clients.