MediView Announces Study Results Demonstrating High Registration and Targeting Accuracy of Augmented Reality-Based Navigational Guidance System
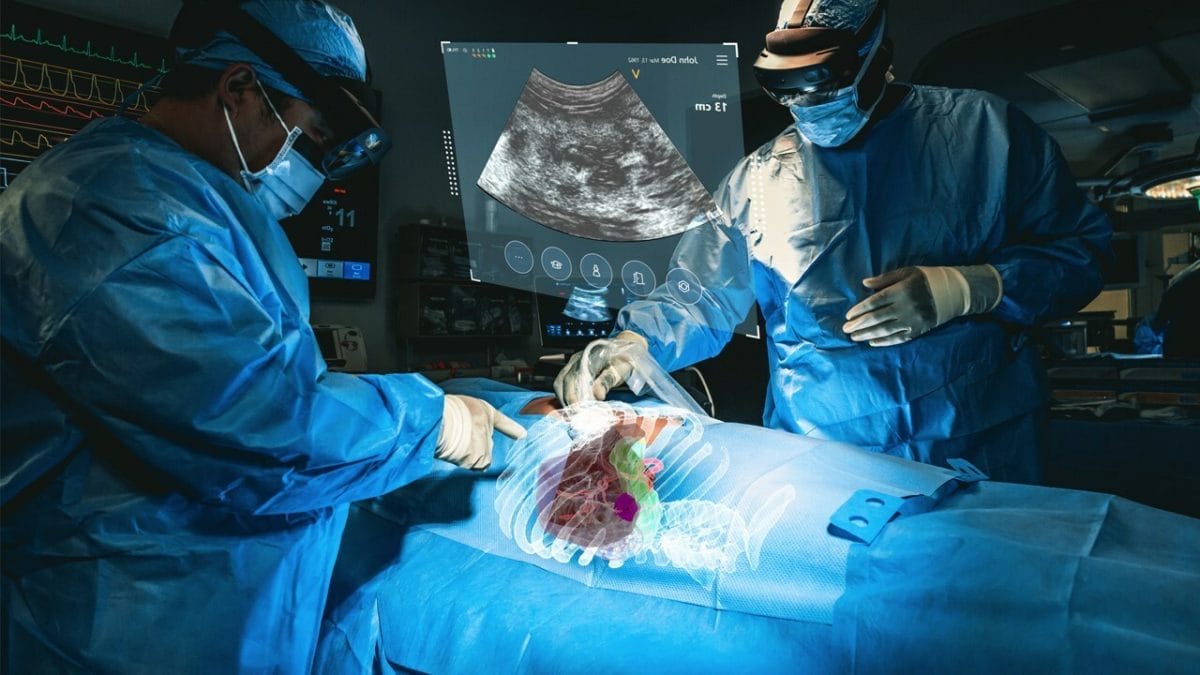
MediView notes the system, which has received 510(k) clearance from the U.S. Food and Drug Administration and is currently in clinical use, is intended to be used adjunctively for minimally invasive ultrasound guided needle-based procedures for soft tissue and bone.