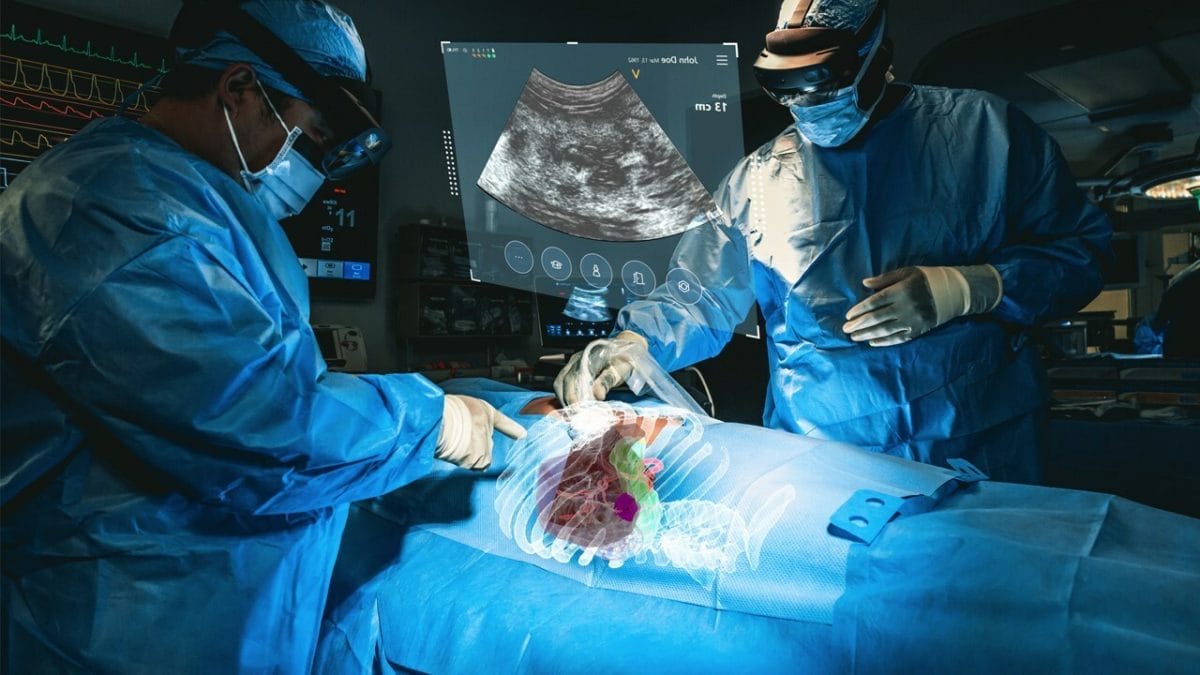
MediView XR Study
MediView XR, Inc., a leading clinical augmented reality (AR) med-tech company, announced today study results of its XR90 AR-based surgical visualization and navigation system, published recently in the Journal of Medical Imaging.
The system, which has received 510(k) clearance from the U.S. Food and Drug Administration and is currently in clinical use, is intended to be used adjunctively for minimally invasive ultrasound guided needle-based procedures for soft tissue and bone.
Gaurav Gadodia, MD, interventional radiologist at VIR Chicago and co-author of the study
“Sub-centimeter accuracy is required for many minimally invasive percutaneous procedures like biopsies and ablations. As such, any device aimed at helping improve these procedures in any way must prove accuracy before other benefits can be evaluated or deemed efficacious. This study sought to grow the evidence that MediView’s novel augmented reality solutions is accurate, and thus may support use in the procedural suite to help facilitate improved visualization and guidance.”
MediView’s XR90 system overcomes the limitations of two-dimensional imaging by providing physicians with 3D “X-Ray vision” during procedures – the ability for the clinician to see their patient’s comprehensive internal anatomy in 3D, including bone, tissue, organs, and vasculature. The device displays 3D images of the patient’s own anatomy based on their CT imaging and fuses that CT with live ultrasound to perform minimally invasive procedures, such as biopsies and tumor ablations.
In this study, researchers sought to quantify the targeting and image fusion registration accuracy of MediView’s multimodal AR image guidance platform for needle-based procedures in a cadaver model implanted with targets mimicking cancerous tumors inside the liver. The AR system was used by three operators to visualize and navigate to the targets with tracked needles in 36 procedures performed on four targets.
Accuracy
Researchers evaluated the accuracy of the system using two methods: 1) Target registration error (TRE), a measurement of the main system error, defined as the Euclidean distance between the needle tip and the target after the needle has been placed; and 2) image fusion registration error (IFRE), a measurement of multimodal registration error between different imaging modalities such as CT and ultrasound (US). Per the publication, both TRE and IFRE are quantitative measurements that reflect the accuracy of a 3D needle guidance system.
Researchers determined statistically significant TRE and IFRE measurements, with mean IFRE 4.4 mm (H0: μ=5mm, P<0.05) and mean TRE 2.3 mm (H0: μ=5mm, P <0.00001). Per findings of this study, the accuracy metrics provided evidence that the AR needle guidance system can be used to reach targets with the precise degree of accuracy required for clinical applications, when used as an adjunct to standard-of-care imaging.
MediView XR, Inc. is a Cleveland Clinic portfolio company with core technology originally created at Cleveland Clinic. “Having a mean TRE of under 5mm establishes that MediView’s XR90 system has the potential to be used for targeting within the borders of the smallest treatable tumors, which is oftentimes observed during clinical practice,” said Charles Martin III, MD, study investigator and staff interventional radiologist at Cleveland Clinic. “The ability of this AR-based navigational guidance system to reliably facilitate needle trajectory planning and guidance for percutaneous procedures could potentially facilitate better clinical performance.”
MediView’s XR90 technology will be showcased at booth #1105 at the upcoming Society of Interventional Radiology conference in Salt Lake City, March 23-28, 2024.