By Randy Horton is Chief Solutions Officer at Orthogonal,
Just like hardware medical devices, Software as a Medical Device (SaMD) are grouped together across the FDA’s 16 medical specialty panels. In our firm’s journey to create a comprehensive list of all FDA-cleared SaMD, this article takes a quick look at five interesting SaMD from the Cardiovascular panel that were cleared by the FDA in 2023. The common thread among these recently cleared SaMD is a focus on remotely monitoring patients and alerting them or their clinicians when irregular heart rhythms or dangerous heart events occur.
Orthogonal estimates that our list of SaMD may only be ~10% complete at this point. Help us improve our list by submitting any SaMD you are aware of using this form or by emailing us at SaMD-list@orthogonal.io.
Remote monitoring of ICU patients: T3 Platform Software
Company: Etiometry
Decision Date: July 7th, 2023
FDA Summary: K223578
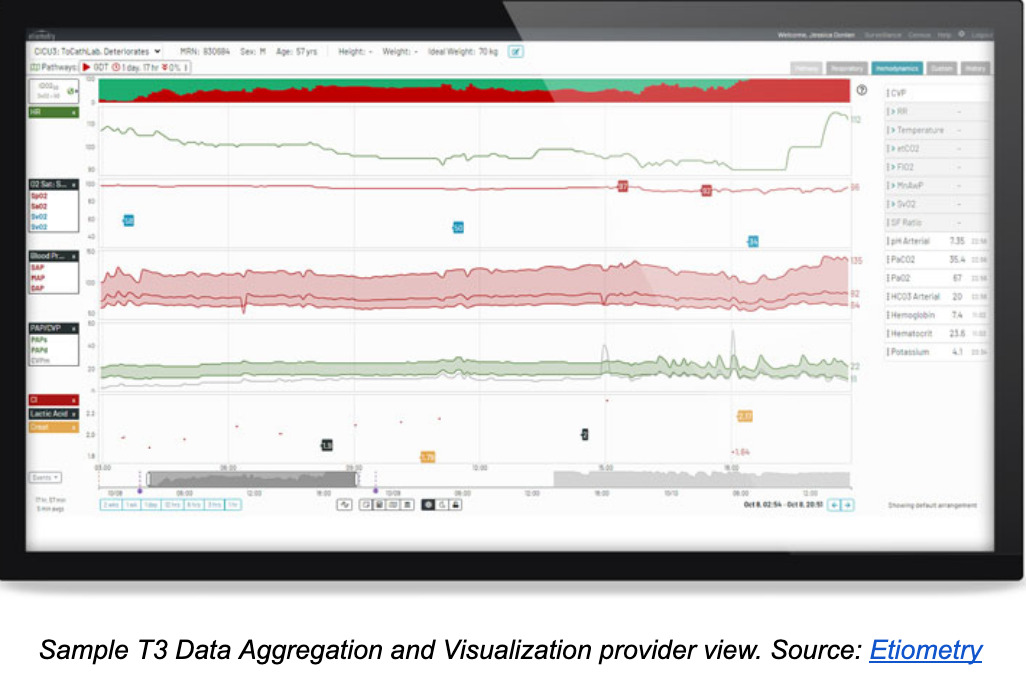
Clinical decision support software provider Etiometry received clearance for the latest version of their intensive care software solution known as T3 – Tracking, Trajectory and Trigger.
This updated SaMD, first cleared in 2015, is composed of two parts. The T3 Data Aggregation and Visualization module collects, presents and stores cardiac data from multiple sources in an ICU setting. Among the kinds of data the T3 system can pull from sensors and medical devices are a patient’s arterial blood pressure, cardiac output, oxygen saturation, pulse rate and atrium pressure. Clinicians can use this software to remotely view a patient’s status in near real-time.
The second part of the T3 Platform is the T3 Risk Analytics Engine, which calculates the probability of life-threatening cardiac conditions and directs clinicians to intervene. The engine examines physiologic data and laboratory measurements received by the Data Aggregation and Visualization module to monitor for inadequate delivery of oxygen, inadequate ventilation of carbon dioxide, presence of acidemia and presence of hyperlactatemia.
Heart monitoring on your schedule: Samsung Galaxy Watch and Apple Watch’s Irregular Heart Rhythm Notification Features
ECG Monitor Application with Irregular Heart Rhythm Notification
Company: Samsung
Decision Date: May 2nd, 2023
FDA Summary: K230292
Irregular Rhythm Notification Feature
Company: Apple
Decision Date: July 21st, 2023
FDA Summary: K231173
Tech giants Apple and Samsung not only compete with their iPhone and Galaxy smartphones but also with their Apple Watch and Galaxy Watch smart watches. The Apple Watch was the first to add a built-in electrocardiogram (ECG) and heart monitoring feature in 2018; Samsung wasn’t far behind with their own ECG app in 2020. Both watches perform a single-channel ECG and store recordings on a companion health app on the user’s smartphone.
In 2023, Samsung received clearance for their ECG watch app with an irregular heartbeat notification feature, bringing the Galaxy Watch’s app in line with the Apple Watch’s. This feature passively listens to the user’s heart rate and pulse and alerts when an irregular heartbeat could indicate an occurrence of atrial fibrillation.
Apple’s 2023 clearance for their app allows them to implement a Predetermined Change Control Program (PCCP) for their Irregular Rhythm Notification Feature algorithm. PCCP is a new regulatory tool that names very specific modifications that Apple can make to their algorithm/device without filing for another clearance from the FDA.
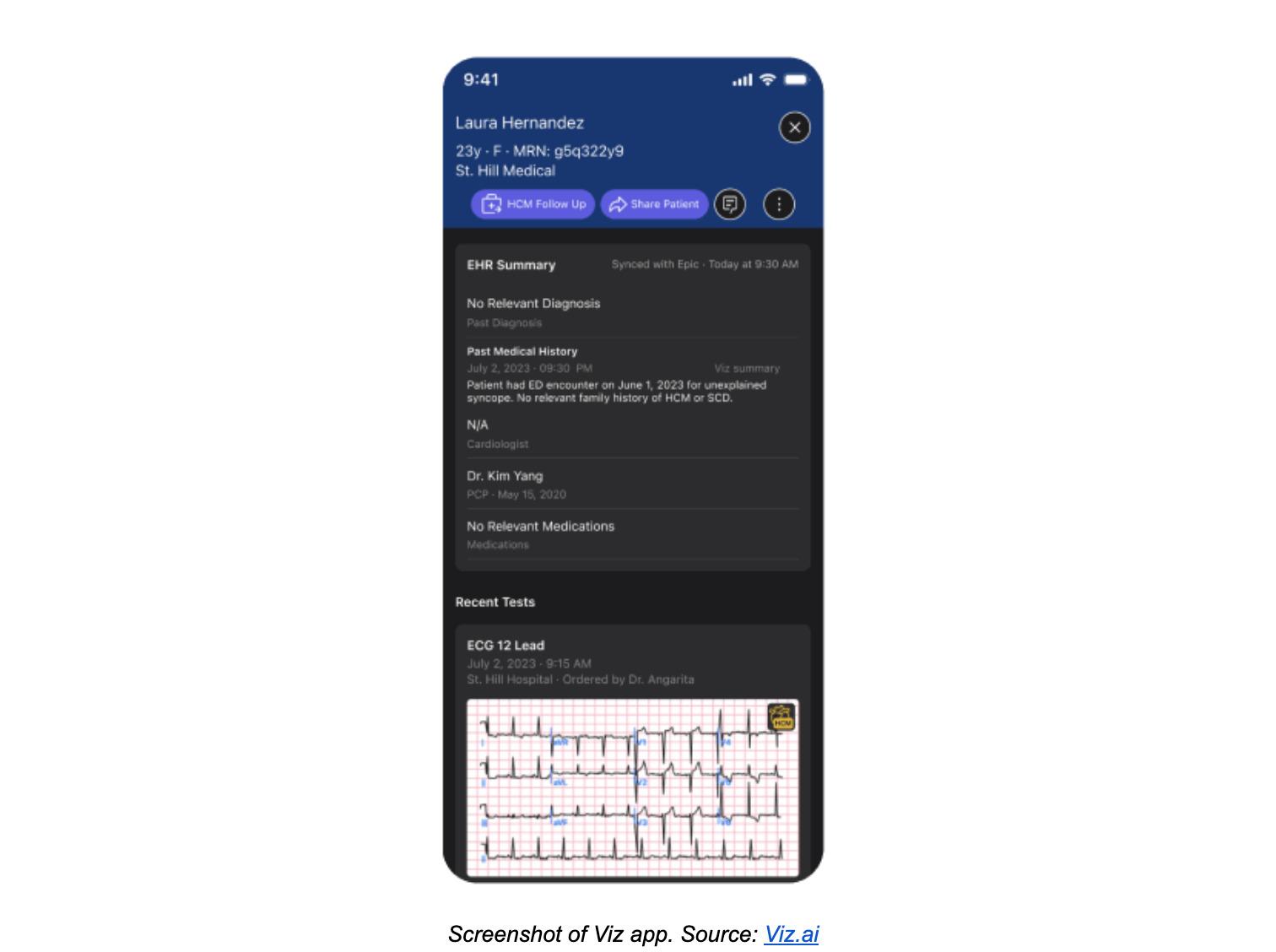
Machine Learning cardiovascular software: Viz HCM
Company: Viz.ai
DEcision Date: August 3rd, 2023
FDA Summary: DEN230003
The first FDA-cleared device of its kind, Viz HCM is a cardiovascular Machine Learning-based notification software. Its AI algorithm reviews 12-lead ECGs across an entire hospital system to identify signs of hypertrophic cardiomyopathy (HCM), a heart disease that causes the thickening of the heart muscle and can lead to a sudden and early death. Once a patient’s ECG is flagged for HCM, Viz’s mobile app platform alerts the appropriate clinician, who can view the ECG reports and follow up with the patient.
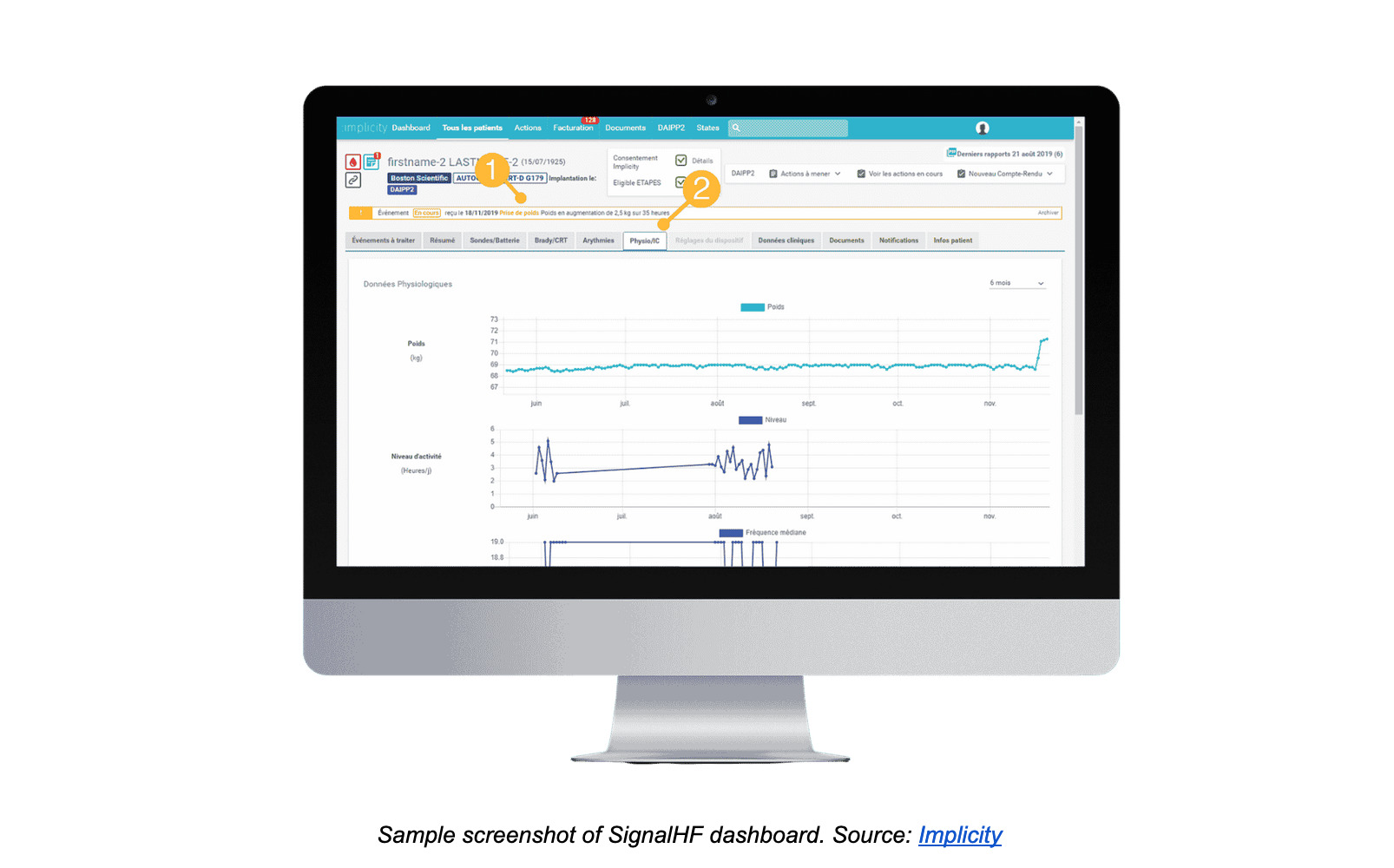
Scoring heart health: SignalHF
Company: Implicity
Decision Date: October 25th, 2023
FDA Summary: K230842
SignalHF’s algorithm scores the risk of heart failure by sampling data from a patient’s compatible cardiac implantable electronic devices and electronic health record data. Based on its calculations, SignalHF can predict a potential heart failure around thirty days before it occurs, long before the patient is hospitalized. This enables clinicians to preventatively treat patients at home and avoid the health consequences and costs of a heart attack.
Conclusion
Whether reacting to atypical heart events or predicting the likelihood that they’ll occur, SaMD can play an important role in cardiovascular care. We anticipate that the FDA will clear more cardiovascular SaMD that leverage AI and Machine Learning algorithms to improve their predictive abilities, enabling clinicians to treat patients before adverse heart conditions occur.
This selection of SaMD was taken from Orthogonal’s Ultimate Running List of every FDA-cleared SaMD. Even with over 500 SaMD cataloged, our journey to record all SaMD on the market in the U.S. is far from over. Help us complete our list by filling out this form or emailing us at SaMD-list@orthogonal.io.
Editor’s Note
Randy Horton is Chief Solutions Officer at Orthogonal, a software consulting firm that improves patient outcomes faster by helping MedTech firms accelerate their development pipelines for Software as a Medical Device (SaMD), digital therapeutics (DTx) and connected medical device systems. Orthogonal makes that acceleration happen by fusing modern software engineering and product management tools and techniques (e.g., Agile, Lean Startup, User-Centered Design and Systems Thinking) with the regulated focus on device safety and effectiveness that is at the heart of MedTech.
Horton serves as Co-Chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS and the Human Factors and Ergonomics Society (HFES).