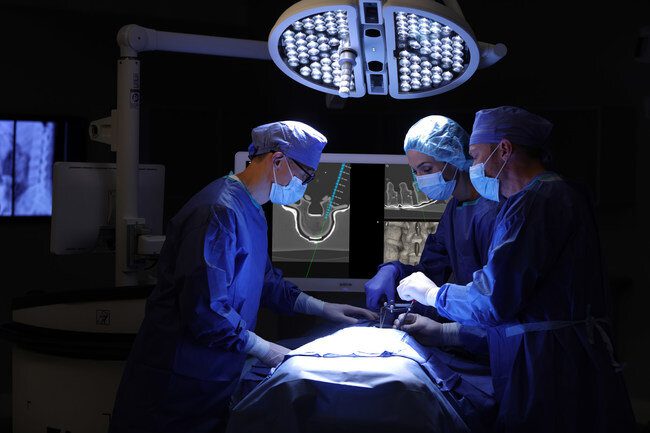
7D Surgical, based in Toronto, Canada, is expanding its footprint into the European Market with multiple units already placed. 7D Surgical has also completed several spinal surgical cases and tissue labs utilizing the FLASH™ Navigation System in Germany and Switzerland.
7D Surgical notes since sales first commenced in 2018, 7D Surgical has surpassed 50 units sold in just over two years.
7D Surgical Beau Standish, Chief Executive Officer said, “Aggressively expanding our international footprint has been a strategic priority for the company in 2020.” He added, “We have now successfully launched in the United States, Canada, Singapore, Australia, New Zealand, and the Caribbean and are excited to enter into the European market to share our machine-vision technology with the global healthcare community.”
7D Surgical has developed technology, similar to GPS navigation in self-driving vehicles, to create a three-dimensional image for surgical navigation in just seconds, resulting in shorter and more efficient spinal and cranial procedures. The FLASH™ Navigation System uses only visible light, eliminating the patient and staff exposure to intraoperative radiation. It is the only approved image guidance system that utilizes this novel and proprietary camera-based technology, coupled with machine-vision algorithms to eliminate the long-standing frustrations with legacy surgical navigation platforms. The speed, accuracy, and efficiency combined with a fundamentally streamlined surgical workflow provides significant economic value and harnesses the true potential of image guidance – all while enabling a safe and radiation-free surgical environment.
In addition to its rapid global expansion, 7D Surgical has achieved numerous milestones in recent months, including surpassing its 13,000th pedicle screw guided with the FLASH™ Navigation System. The company also completed its first pediatric installation in the United States, adding to their global footprint of pediatric hospitals. The FLASH™ Navigation System is ideally suited to deliver navigation technology that is safe and radiation-free for this patient population.
“I have been following navigation for many years, but never felt right about the technology out there because it was bulky, cumbersome, and slow. The 7D System completely changed that and has great, cutting-edge technology that is simple and efficient,” said Dr. Chris Comstock, pediatric spine surgeon at Driscoll Children’s Hospital in Corpus Christi, Texas. “I’ve used it on very complex deformity cases, and it worked beautifully. It is very compact, easy to mobilize, surgeon friendly to operate, and extremely fast and accurate… and the fact that it eliminates the radiation exposure to myself and my patients is a game-changer.”