- CharmHealth Advances Its AI Strategy With MCP Server
- Evinova Announces Strategic Collaborations with Astellas, AstraZeneca and Bristol Myers Squibb Advancing Its AI-Native Platform to Accelerate Global Clinical Development
- ProSomnus Secures $38 Million Strategic Investment from Catalio Capital Management to Scale Smart Sleep Medicine™
- Simpson Interventions Announces Initial Series C Close and Rebrands as Elumn8 Medical
- Johnson & Johnson Boosts U.S. Presence with $1 Billion+ Investment in Next‑Gen Cell Therapy Facility
Stay Ahead in Medical Technology
Medical Device News Magazine takes pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
CharmHealth Advances Its AI Strategy With MCP Server
By embedding AI as core infrastructure rather than an add-on, CharmHealth is advancing a more practical, governed approach to AI adoption with the EHR
Primera Selects Cenevo’s Labguru to Transition from Paper to Digitalized Workflow
“Beyond our specialized CRO solution set, our client-facing team has extensive real-world CRO experience,” said Keith Hale, CEO of Cenevo. “Our tailored solution and industry experience will help guide Primera through the steps they need to fully embrace automation and digitalization in their highly specialized environment.”
Widex Names U.S. Ski Jumper Estella Hassrick an Official Brand Ambassador, Inspiring a New Generation to Live Boldly
Widex today announced the addition of U.S. Ski Jumper Estella Hassrick as its newest brand ambassador. At just 19, ESTELLA Hassrick has already distinguished herself
Baird Medical Accelerates Global Commercial Roadmap with Strategic Clinical Education Initiatives in the U.S. and Japan
Simultaneously, Baird Medical solidified its presence in Asia through a high-profile workshop at Showa Medical University in Yokohama
Clinical Trials
Vektor Medical Announces EP Europace Publication: vMap-Guided Ablation Associated with Improved One-Year Arrhythmia-Free Survival in Unstable Ventricular Tachycardia Patients
80% One-Year Arrhythmia-Free Survival Achieved Without Increased Procedure Time, Fluoroscopy, or Complications
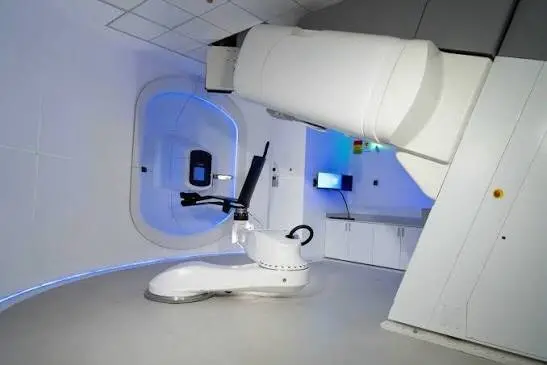
P-Cure Announces 1st Ever Prospective Clinical Results of Gantry-Less Upright Proton Therapy for Lung Cancer Re-Irradiation
The P-Cure center is the first and only facility in the world to routinely deliver proton therapy to patients using its upright technology

Study highlights GLP-1 companion effects, underscoring capability of Brightseed’s AI platform to identify and launch clinically-validated, natural health innovations
The study was published in the peer-reviewed journal Bioactive Compounds in Health and Disease
Biotechnology News
Evinova Announces Strategic Collaborations with Astellas, AstraZeneca and Bristol Myers Squibb Advancing Its AI-Native Platform to Accelerate Global Clinical Development
Each partner company has opted in to share operational data with Evinova, enabling the platform to provide benchmarks and smarter recommendations intended to further accelerate clinical trials and improve patient outcomes
Cenevo Advances Toward Agentic Labs with Two New AI Agents
The new agents are the AI Protocol Conversion agent and the AI Automation agent.
Circular Genomics Finalizes Exclusive IP Licenses with Leading Global Research Institutions to Advance circRNA Blood Test for Alzheimer’s Disease
Agreements strengthen proprietary foundation, enhance partnering readiness, and support regulatory pathways for next-generation molecular diagnostics in neurology
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

HTM’s next frontier: AI, intelligent operations, and tech-driven resilience in 2026 | By Neil de Crescenzo, Chief Executive Officer at TRIMEDX
In this ever-changing healthcare landscape, prioritizing data-driven decision-making and proactively adopting advanced technologies will position health systems to achieve lasting success in 2026 and the years ahead

Digital Pathology Reaches an Inflection Point as AI Targets Workflow Gaps | By Yair Rivenson, PhD Founder and CEO, Pictor Labs
Laboratories face mounting pressure to modernize imaging, preserve tissue, and reduce sequencing failures as adoption continues to move at a glacial pace

FIZE Medical Launches 5,000-Patient Retrospective Study to Further Establish the benefits of using FIZE kUO in managing ICU patients’ Hemodynamic status
By continuously capturing renal function patterns, the study aims to demonstrate how FIZE kUO® can provide data for early signs of instability that are often missed by intermittent measurements
Executives on the Move

Michael Clay Named COO at Caidya
To drive operational excellence and advance multi-regional clinical development
First Ascent Biomedical Opens FPM Cancer Lab in Miami; Welcomes World-Class Clinical Leadership
Miami lab is now accepting patient and physician referrals. Eligible Florida residents and colorectal cancer patients nationwide may qualify for limited sponsored programs covering the cost of testing

Howard Brooks Appointed to the Immorta Bio Strategic Advisory Board
Mr. Brooks will work with Immorta Bio’s leadership team as the Company advances its first-in-class, dual-platform approach to addressing aging biology
FDA Medical Device Updates
Medtronic receives FDA clearance for Stealth AXiS™ surgical system | 1st Integrated planning, navigation and robotics platform for spine surgery
The Stealth AXiS™ system is cleared for spine procedures in the United States, with an underlying architecture designed to support future cranial and ENT applications, pending 510(k) clearance.
Medtronic Diabetes expands access to comprehensive insulin delivery solutions following Medicare coverage and new FDA clearances
These milestones include Medicare access for the MiniMed™ 780G system paired with the Instinct sensor, made by Abbott, FDA clearance for the system’s use with ultra rapid‑acting insulins, and clearance of the MiniMed™ 780G system for use with the Instinct sensor for insulin-requiring type 2 diabetes
Proprio Reports: 4th FDA Clearance Highlights Growing Shift to 3D Measurement in Spine Care
Picasso Experience Expands Surgeon-Controlled, Radiation-Free Registration Workflows for Real-Time Intraoperative Spinal Alignment Measurements
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
ProSomnus Secures $38 Million Strategic Investment from Catalio Capital Management to Scale Smart Sleep Medicine™
Strategic alliance focuses on scaling data-integrated sleep solutions to meet surging clinical and consumer demand

Simpson Interventions Announces Initial Series C Close and Rebrands as Elumn8 Medical
Series C financing supports continued clinical development of the Acolyte™ Image-Guided coronary chronic total occlusion platform, an FDA Breakthrough Device

Johnson & Johnson Boosts U.S. Presence with $1 Billion+ Investment in Next‑Gen Cell Therapy Facility
Advanced manufacturing site will utilize cutting‑edge cell therapy technologies to help deliver the company’s portfolio of transformational medicines
GE HealthCare announced a new approximately $35 million expansion to a previous contract with the BARDA
The expansion is structured as a cost-share between BARDA and GE HealthCare, with BARDA providing the majority of funding
Market Reports
Advancements In Imaging

PreciseOnco research consortium awarded EUR 14.9 million IHI grant to drive breakthroughs in precision cancer treatment
Co-funded by the EU’s Innovative Health Initiative (IHI), the Philips-coordinated research consortium will combine advanced medical imaging, robotic guidance technologies, and AI to standardize and enhance the precision of minimally invasive cancer treatments
New Peer-Reviewed Study Validates AISAP’s AI Ability to Detect Major Heart Disease from a Single Ultrasound View
Retrospective Analysis of Over 120,000 Echocardiographic Studies Provides Clinical Evidence of Diagnostic Accuracy for Heart Failure and Valvular Disease
Arineta Reports | Yuma Cardiology Practices Partner to Bring Advanced Imaging to Underserved Patients in Arizona with Arineta’s SpotLight™
The joint effort expands access to advanced cardiac imaging with the highest performance CT in Arizona for high-risk and underserved populations, including seniors, Native American communities, and lower-income residents.
Hospitals In the News

Good Samaritan Medical Center Successfully Performs Focal Cryoablation for Prostate Cancer Using Advanced KOELIS Trinity® Fusion and Varian Cryoablation Technology
Good Sam and Dr. Jue are the only hospital and surgeon from Orlando through Miami to perform the surgery

Kauvery Hospital, Alwarpet Performs One of the World’s First TAVR-in-TAVR-in SAVR with Bioprosthetic Valve Fracture in 78-Year-Old
The advanced transcatheter intervention was undertaken to treat a repeatedly failing heart valve in a patient with a long and challenging history of aortic valve disease
Texas Cardiac Arrhythmia Institute First in U.S. to Implant FDA-Approved Novel Defibrillation Lead
The new technology is the world’s smallest, catheter-delivered defibrillation lead
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Hope For The Warriors Awarded $3 Million Grant from Lilly Endowment Inc.
$3 million investment strengthens foundation for the next generation of military family support
ACC Announces Inaugural Fellow for the Thad and Gerry Waites Rural Cardiovascular Research Fellowship
The new award provides up to $70,000 in funding to a Fellow-in-Training or an early-career cardiologist
AAOS Establishes Orthobiologics Registry to Advance Evidence-Based Orthobiologics Research
Pilot program addresses critical evidence gap in emerging regenerative medicine treatments for 32.5 million Americans with knee osteoarthritis
Healthcare: Understanding the Business Side of the Industry and Its Implications

How Executive Skills Transform Local Governance
Something interesting happens when executives apply their skills to neighborhood challenges. They don’t just help communities. They develop themselves in ways their professional contexts couldn’t provide.

Medical Postgraduate Courses: A Complete Guide
Medical Postgraduate Courses: A Complete Guide for Aspiring Specialists Medical postgraduate courses are advanced academic and clinical programs pursued after completing an undergraduate medical degree

How to Successfully Navigate a Dental Practice Ownership Transfer
A smooth transition comes from planning, clear communication, and the right support. When each step is handled with care, the dental practice stays strong and trusted.

Streamlining Financial Operations With E-Invoicing Solution Providers and Automated Payroll Processing
Organizations now turn to e-invoicing solution providers and automated payroll systems to address these challenges, though many struggle to identify which technologies deliver measurable returns. Understanding how these tools transform financial workflows reveals critical advantages that separate efficient operations from those still trapped in outdated processes
Healthcare Careers

Innovative Careers in Healthcare Leadership
The future of healthcare leadership will be defined by those who embrace continuous learning, adaptability, and innovation

Top Ways to Build a Successful Career in the Field of Psychology
A fulfilling role in the mental health space takes more than a degree. It takes strategy, clarity, and a commitment to steady progress.

Why Choose Hands-On, Real-Patient Training for Your Dental Implant Career?
Hands-on, real-patient training bridges that gap, providing invaluable experience that builds both confidence and capability. For dentists serious about growing a successful implant practice, this approach is not just beneficial—it’s essential.
Nurses Corner

Nurse Burnout: A Strategic Threat Hospitals Can’t Afford to Ignore
Burnout isn’t just “being tired.” It’s a chronic state of emotional, mental, and physical exhaustion caused by prolonged stress.

Nurses | How To In Shape
Many nurses manage to stay in great shape, both physically and mentally—despite the challenges

Best Clothing for Nurses: Comfort, Function, and Style
Choosing the right attire can significantly impact a nurse’s performance, safety, and overall well-being. Here’s a detailed guide to the best clothing for nurses