Accelus
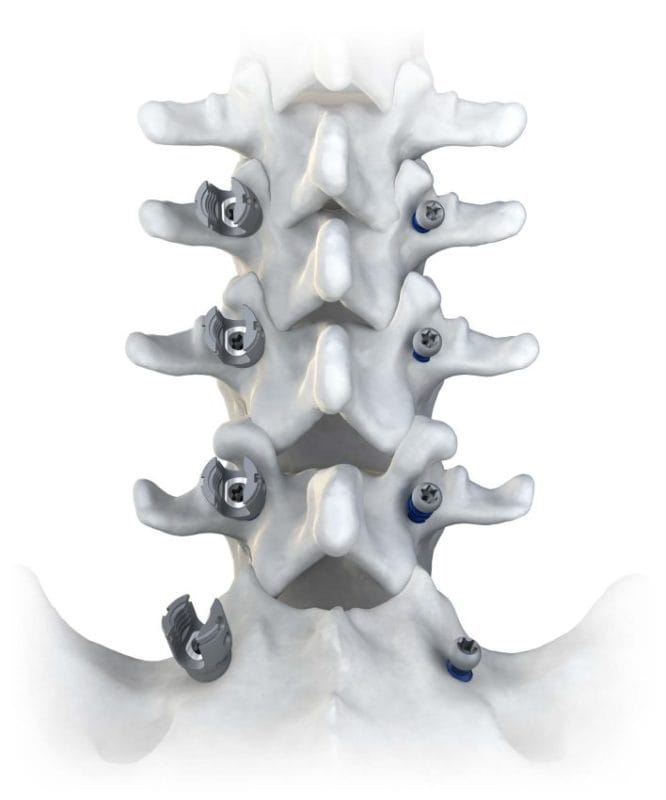
Accelus, a privately held medical technology company committed to becoming the global market leader in expandable spinal implant technologies, today announced the launch of the LineSider® Modular-Cortical System. Designed for greater procedural visibility, versatility and efficiency, the modular screw design provides surgeons with enhanced visualization by allowing the placement of screw shanks early in the procedure, followed by modular tulips and rods at the end.
Expansion
This expansion to Accelus’s LineSider Spinal System product portfolio is designed to meet the needs of surgeons who use a modular pedicle screw system from either a standard open approach or a medial-to-lateral cortical approach. The modular tulip heads feature a slim profile and low height to facilitate a mid-line approach, and the modularity of the system provides surgeons with versatility in their surgical approach, along with the ability to customize the construct to address the patient’s specific anatomy and pathology needs.
LineSider Modular-Cortical System
“Incorporating the new LineSider Modular-Cortical System into my case workflow was easy due to the innovative and user-friendly design of the system,” said Jay K. Shah, D.O., MBA, attending orthopaedic spine surgeon, NewYork-Presbyterian Hospital and Clinical Instructor in Orthopaedic Surgery, Weill Cornell Medical College. “The purchase of the screw shanks coupled with the quality of the modular tulips makes this system one of the top runners in the industry.”
The LineSider Modular-Cortical System is available in open modular and cortical modular sets, with both sets designed to be compatible with existing LineSider instrumentation. The open modular set features dual-lead screw shanks and expands the implant offerings of the legacy
LineSider open Sets
The cortical modular set was designed to facilitate an improved midline approach, offering dual-to-quad lead shanks for increased purchase in cortical bone. The shanks in each system are available in cannulated and solid options. The cortical modular set includes a new midline lumbar retractor with retractor blades in 23mm and 46mm widths, further augmenting the system’s efficacy and Accelus’s commitment to providing comprehensive and adaptable spinal surgery solutions.
“I highly recommend utilizing the LineSider Modular-Cortical System to complement the FlareHawk expandable interbody fusion device,” said Dr. Ryan Martyn, orthopedic spine surgeon at Spine Colorado. “There are very tight tolerances in the machining, so there is no toggle between the shank and the driver when inserting the screw, and the modularity of the system allowed me to do my facetectomies much easier with no tulip in the way.”
In addition to in situ assembly, the LineSider Modular-Cortical System can also be utilized in a pre-assembled fashion, giving surgeons the flexibility of a non-modular screw system as needed. The LineSider Modular-Cortical System builds on Accelus’s legacy of developing adaptable, surgeon-centric solutions. To date, more than 10,000 LineSider screws have been implanted in the United States, and 20,000+ FlareHawk interbody fusion devices have been implanted in 23 countries worldwide.
Accelus President and Chief Executive Officer Kevin McGann
“The LineSider Modular-Cortical System exemplifies our commitment to elevating the surgical experience and improving patient outcomes. The system not only enhances surgical visibility and flexibility but also maintains our previously set standard for reducing the number of LineSider trays required, helping to optimize O.R. setup and procedural workflow.”