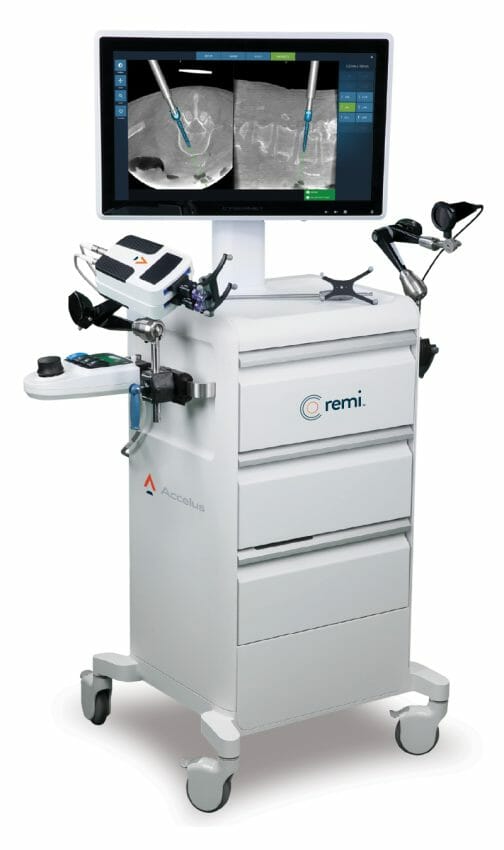
Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced Vail-Summit Orthopaedics and Neurosurgery (VSON) as the first ambulatory surgery center (ASC) to acquire the Remi Robotic Navigation System, a comprehensive robotic targeting and navigation platform that provides robotic-assisted pedicle screw placement for surgeons performing lumbar spine fixation.
The Remi Robotic Navigation System was designed to address the limitations of current spinal robotic systems and features the same accuracy with a smaller footprint, efficient procedural workflow, shorter setup and teardown times, and more economically accessible pricing. Remi’s Big Eye™ camera technology features a wide angle of capture with flexible table-mounted positioning to reduce line-of-sight occlusion. The system’s robotic targeting arm is also mounted to the surgery table, eliminating the operating room footprint required by traditional robotic systems. Its surgeon-centric software is designed around spine surgery workflows to help anticipate next actions and enhance the system’s ease of use.
“With a much smaller footprint and lower cost than traditional robotic navigation systems, Remi is designed specifically to meet the needs of the ASC,” said Ernest Braxton, MD, MBA, board-certified Neurosurgeon and Chief of Neurosurgery at VSON.
He added, “Remi represents the next generation in spine technology, and I’m confident in the accuracy, efficiency, and safety this enabling technology will provide my patients. I’m also excited about the future evolution of the technology as Accelus works to make the system compatible with 2D imaging and interbody placement.”
Dr. Braxton and VSON will also serve as one of the first Accelus Centers of Excellence in Spine (ACES) preceptorship programs in the nation. The ACES program allows qualified surgeons to meet with Dr. Braxton and learn from his surgical technique for minimally invasive spine surgery and robotic navigation. It also enables surgeons who are considering the Remi Robotic Navigation System to obtain valuable firsthand experience before deciding if it is a technology that would be beneficial to their practice and facility.
“As more procedures shift from hospitals to outpatient facilities, it is important that new technology is created in a way that makes it amenable to the ASC setting, and we are proud to have Dr. Braxton using Remi for his spine procedures at the state-of-the-art Vail-Summit Orthopaedic & Neurosurgery facilities,” said Chris Walsh, CEO and Co-Founder of Accelus.
Walsh noted, “We believe Remi is a perfect fit for ASC use, providing accuracy with a portable footprint and price tag that fit well for these types of facilities. The ability to move a robot from room to room or facility to facility creates massive clinical efficiencies and value.”
Dr. Braxton is also one of the first neurosurgeons to perform robotic-assisted transforaminal lumbar interbody fusion procedures while the patient is awake, which may allow patients to experience a faster recovery.
“For interested patients, awake surgery can be a more respectful, patient-centered experience that is often better tolerated by the patient without the negative side effects often experienced from anesthesia used in traditional spine surgery,” said Dr. Braxton. “Remi works well for my outpatient surgeries and helps ensure my patients benefit from the most advance technology without complicating the procedure.”
Other robotic industry news can be found here.