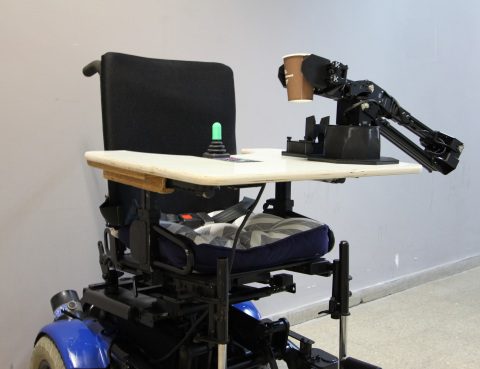
Accenture and Intel are supporting a neuromorphic computing research project led by the Neuro-Biomorphic Engineering Lab (NBEL) at the Open University of Israel in collaboration with ALYN Hospital, Israel’s leading pediatric and adolescent rehabilitation center. Funded by Accenture through its participation in the Intel Neuromorphic Research Community (INRC), the project is focused on developing and testing a wheelchair-mounted robotic arm to assist patients with performing daily tasks.
Researchers from Accenture Labs and Intel Labs are working with researchers from Open University of Israel to build a wheelchair-mounted robotic arm with adaptive controls, using an algorithm developed by Applied Brain Research (ABR) and Intel’s neuromorphic computing hardware. Wheelchair users will be able to independently control the robotic arm to perform daily tasks that require strength and dexterity of arms and hands, such as drinking from a glass, with 50% fewer errors and a 48% improvement in energy efficiency over traditional control methods. The device will undergo clinical testing and evaluation with patients at ALYN Hospital who rely on electric wheelchairs and have motor impairment of their upper extremities.
Studies suggest that wheelchair-mounted robotic arms provide an increased sense of independence for users and that these assistive tools can reduce the need for caregiver time by up to 41 percent. Today, however, the cost of these devices is incredibly high, making them inaccessible to many people who need them.
“The ability of robotic arms to benefit people today is largely limited due to high cost and excessive power consumption,” said Elishai Ezra Tsur, lead project researcher at the Open University of Israel. “This funding from Accenture, along with additional support from Intel and ABR, is allowing us to explore the implementation of adaptive controls on neuromorphic hardware in an effort to address the need for a collaborative, user-friendly, accurate robotic arm at a significantly reduced cost.”