September 8, 2020
Acutus Medical today announced pre-clinical experimental results of a novel diagnostic and therapeutic workflow utilizing the AcQMap® System along with tissue-selective, non-thermal pulse field ablation (PFA) via a proprietary Acutus ablation catheter.
Acutus Medical has been known for its rapid imaging and mapping platform along with access and diagnostic products and now the company is now aggressively pursuing the therapeutic commercial market.
Pulsed field ablation (also known as electroporation) is an emerging ablation modality that distinguishes itself from traditional thermal ablation, such as radiofrequency energy ablation and cryoablation, by delivering therapeutic energy faster and more selectively with minimal collateral damage.
While not fully clinically proven or approved by regulatory authorities for use in cardiac tissue in humans, recent pre-clinical and clinical studies suggest that PFA may enable rapid targeted ablation of cardiac muscle while leaving adjacent tissue, such as nerves, blood vessels and the esophagus, completely unaffected. In addition, PFA does not rely on heat or extreme cold to ablate tissue. The application time required to create a lesion takes less than a second compared to thermal ablation which may take many seconds to minutes at each location.
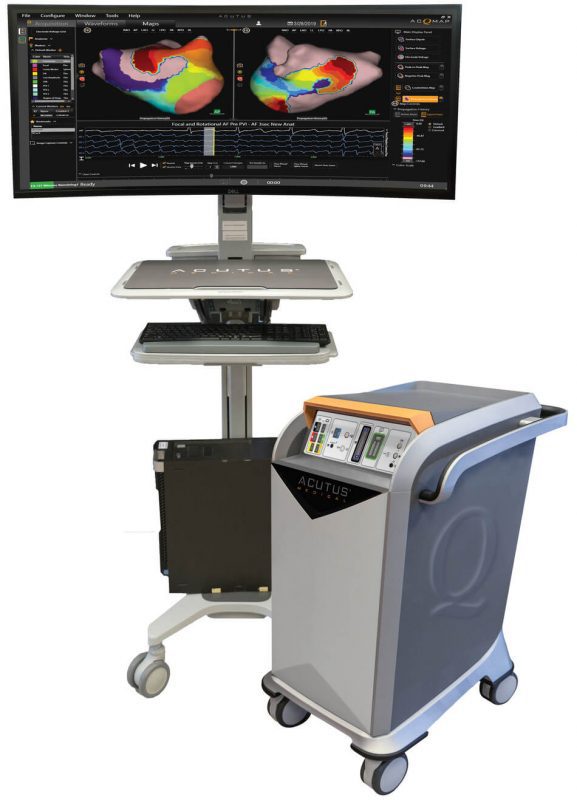
The AcQMap cardiac mapping system, distinguishes itself from conventional contact mapping via ultra-fast ultrasound imaging of cardiac anatomy and information-rich display of actionable diagnostic maps. By coupling the AcQMap System and the potential of PFA, procedure times may be dramatically shortened while enhancing the safety profile.
In a series of pre-clinical experiments, Acutus researchers employed a novel workflow that involved ultrasound imaging of whole-chamber cardiac anatomy and non-contact mapping of electrical activity, followed by delivery of 4 to 8 contiguous ablation lesions in regions of interest via a novel PFA catheter. Following creation of these lesions, non-contact mapping was utilized to assess and confirm the effectiveness of the therapy (successful conduction block) in the treated regions.
In multiple experiments with multiple physician-advisors, this entire whole chamber map / ablate / re-map to confirm block workflow was completed in an average of 12 minutes. Acute ablation efficacy was further confirmed by conventional high-density contact mapping of the treated region with the AcQMap System and a contact mapping catheter.
After a recovery period of 10 days, intra-cardiac re-mapping confirmed lesion durability in the treated experimental model indicating that the ablated regions were irreversibly treated via PFA. Visual observation of cardiac muscle under a microscope further confirmed that the ablated regions were clearly demarcated, spanned the entire tissue thickness and non-cardiac tissue were not affected. More importantly, blood vessels within and near the ablated regions also appeared structurally unaffected.
This post study analysis indicates that durable, non-conductive lesions were created without damage to collateral structures, confirming the hypothesis that PFA may be tissue-selective.
“I am extraordinarily pleased with the effectiveness, speed, and ease-of-use of this integrated system. The initial results of the animal studies are thrilling and provide a robust foundation for moving this program forward toward human trials. I couldn’t be more impressed with the efforts of the Acutus team,” said Dr. Steven Mickelsen an electrophysiologist from the University of Iowa who was intimately involved with this work as a clinical and lead consultant. Dr. Mickelsen is widely regarded as the pioneer of PFA and was instrumental in bringing this therapy to the cardiac ablation space.
“Acutus has consistently been focused on challenging industry standards, thinking outside of the box and investing in highly novel technologies. These initial results combining the use of our novel mapping system with advanced pulsed field ablation technology are quite promising,” commented Vince Burgess, President & CEO of Acutus Medical, Inc.
Acutus Medical with its physician-advisors are preparing to conduct clinical trials to prove the safety and efficacy of PFA in patients with a wide range of cardiac arrhythmias.
Acutus Medical PFA Technology is not for sale in the United States.
Acutus Medical PFA Technology has not been granted CE Mark.