Aerin Medical a company dedicated to providing Ear Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, today announced key events at upcoming annual meetings for the American Rhinologic Society (ARS 2021) and American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNSF 2021).
At the ARS annual meeting, physician investigators will discuss results from two clinical studies evaluating VivAer®, a non-invasive, temperature-controlled, radiofrequency (RF) device, for the treatment of nasal airway obstruction (NAO) caused by nasal valve collapse (NVC):
- “Temperature-Controlled RF Treatment of the Nasal Valve for NAO: A Randomized Controlled Trial” will be presented by Dr. Stacey Silvers of Madison ENT & Facial Plastic Surgery, New York, NY on Saturday, October 2 at 11:15 a.m. PT in Breakout C.
- “Temperature-Controlled RF Treatment of the Nasal Valve for Nasal Airway Obstruction” will be presented by Dr. William Yao from the Department of Otorhinolaryngology-Head and Neck Surgery at McGovern Medical School at The University of Texas Health Science Center at Houston on Saturday, October 2 at 11:22 a.m. PT in Breakout C.
Additionally, the company will host an educational event at AAO-HNSF 2021, “In-Office Advances for Treatment of Nasal Obstruction and Chronic Rhinitis,” on the evening of Sunday, October 3. This symposium will feature Joseph K. Han, M.D., professor, Eastern Virginia Medical School; chief, division of rhinology and endoscopic sinus-skull base surgery and chief of the division of allergy, and Amber Luong, M.D., Ph.D., professor and vice-chair for research, McGovern Medical School at The University of Texas Health Science Center at Houston. They will discuss clinical evidence and case studies regarding newer, non-invasive solutions to treat the common conditions of nasal airway obstruction and chronic rhinitis. Diana, a VivAer patient who suffered from undiagnosed nasal obstruction for years, will join Drs. Han and Luong to share how the condition impacted many aspects of her life and her journey to treatment and relief. For more information about this event or Aerin Medical’s other activities at these meetings, contact info@aerinmedical.com.
Aerin Medical is pleased to support the educational goals of specialty societies and will also be providing information about the company’s portfolio of products, including VivAer and RhinAer® through exhibits at booth #S3 and #1223 for ARS 2021 and AAO-HNSF 2021, respectively. RhinAer is a non-invasive, temperature-controlled RF technology used to disrupt nerve signals that cause chronic rhinitis symptoms.
About VivAer
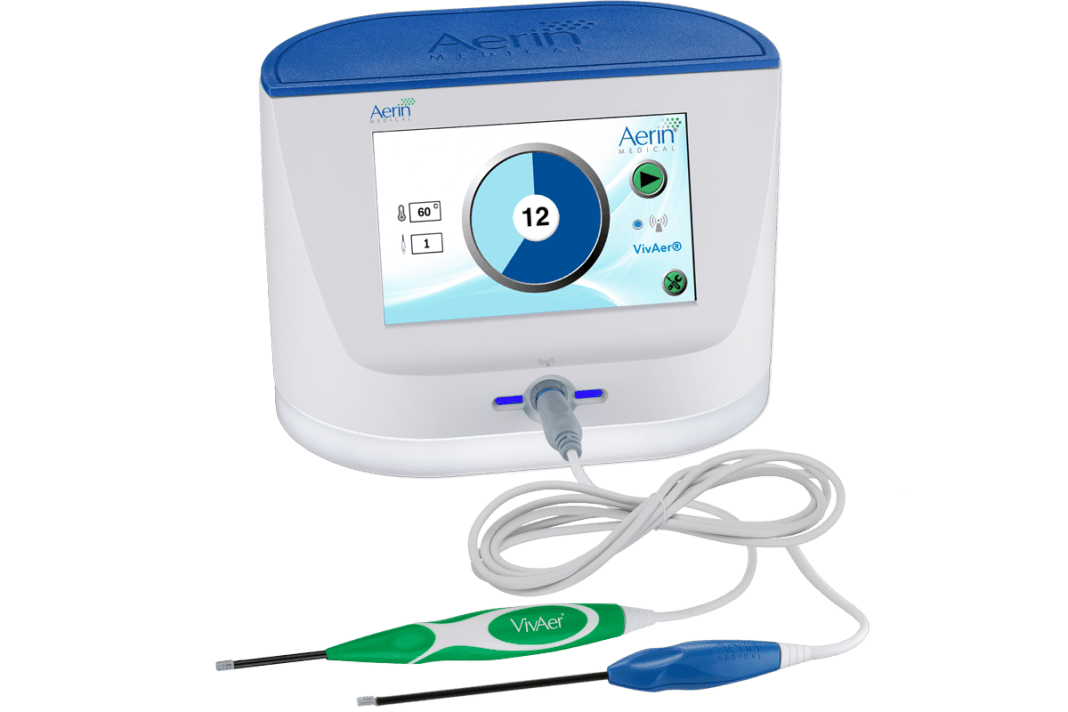
VivAer is a non-invasive technology that uses patented, temperature-controlled radiofrequency energy and is clinically demonstrated to provide long-term relief from nasal obstruction. VivAer features a thin, wand-like stylus that attaches to a console. The stylus is inserted via the nostril to gently remodel the nasal tissue and improve airflow. It is a single solution that is used to treat the lateral nasal wall; it can also treat the turbinates and septal swell bodies that contribute to NAO. VivAer does not involve any cutting, freezing, or removal of nasal tissue or bone. Treatment with VivAer may be performed during a routine office visit with local anesthesia. Patients typically experience little discomfort with minimal to no downtime, and are often able to return to normal activities immediately following treatment.* The VivAer Stylus received CE Mark in 2016 and FDA 510(k) clearance in December 2017.
About RhinAer
Using temperature-controlled, radiofrequency technology, RhinAer features a thin, wand-like stylus that is inserted via the nostril to deliver precise therapeutic benefits, while sparing surrounding tissues. RhinAer directly disrupts the posterior nasal nerve that triggers excessive mucus production, treating an underlying cause of chronic rhinitis. RhinAer provides ENT physicians with a comprehensive solution for the treatment of chronic rhinitis, addressing sources of rhinorrhea (runny nose) and congestion. The procedure can be performed with a local anesthetic during a routine office visit, with no incisions, minimal to no downtime, and little discomfort. RhinAer received FDA 510(k) clearance in December 2019 and CE Mark in 2020.