AliMed, Inc., a Massachusetts-based medical supply manufacturer and distributor, has been awarded a national group purchasing agreement for pediatric straps and positioners with Premier, Inc., and their Performance Group, Kiindo™.
Effective June 1, 2022, the new agreement allows Premier members, at their discretion, to take advantage of special pricing and terms pre-negotiated by Premier for pediatric straps and positioners.
“AliMed is committed to serving the pediatric community with high-quality healthcare products designed specifically for their needs,” said Adrienne Hamilton, Director of Marketing at AliMed. “We are excited about this agreement and look forward to working with Premier members to help them realize cost savings, improve efficiencies, and support better quality outcomes across the pediatric continuum of care.”
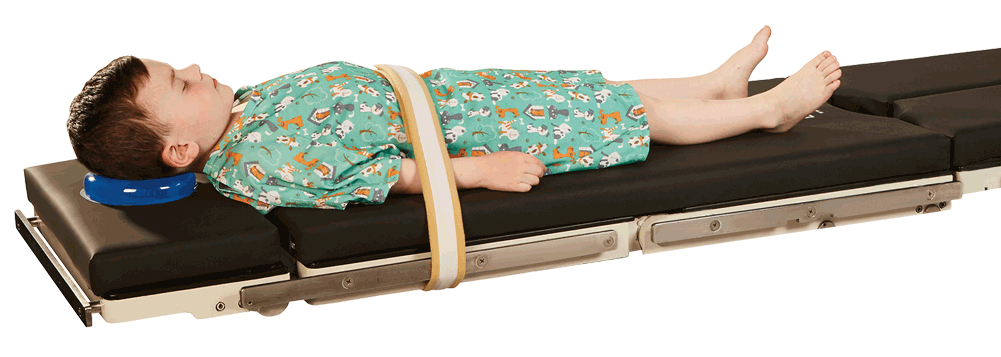
With many products tailored to the unique needs of children from neonatal to adolescents, the AliMed contract includes imaging and surgical straps that provide support and stabilization without damaging delicate pediatric skin, surgical positioners appropriately sized to help relieve pressure from head to foot during procedures, and rehab positioners that aid with strengthening motor skills, balance, mobility, feeding, and more.
Premier is a leading healthcare improvement company, uniting an alliance of approximately 4,400 U.S. hospitals and 250,000 other providers to transform healthcare. With integrated data and analytics, collaboratives, supply chain solutions, consulting, and other services, Premier enables better care and outcomes at a lower cost.
