January 21, 2021
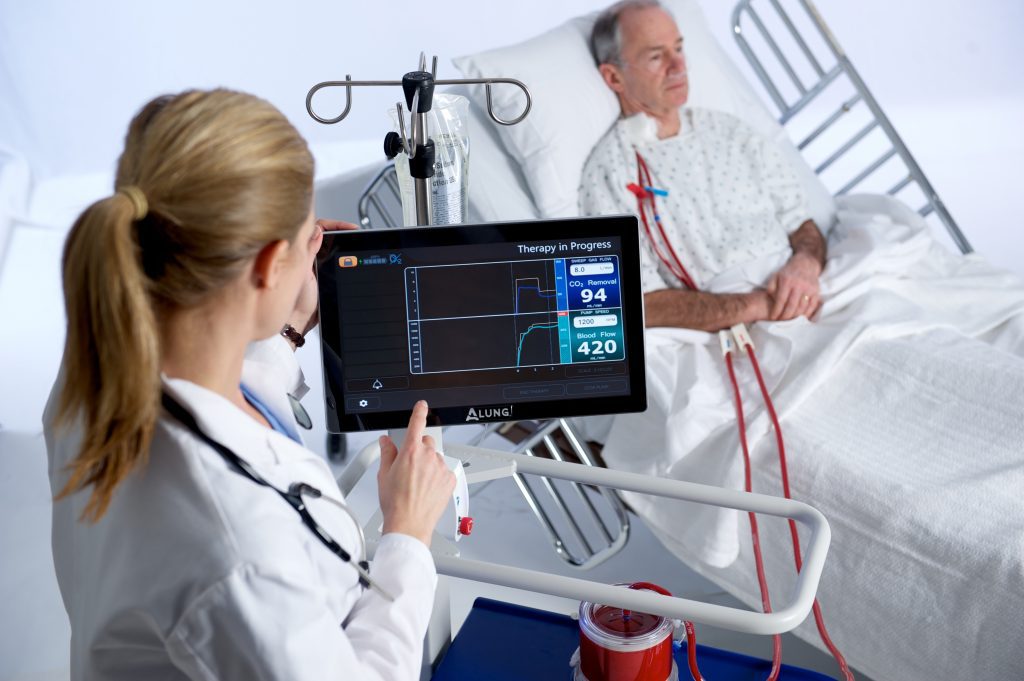
ALung Technologies, Inc. announced that it has now treated more than 75 COVID-19 patients, and that it is experiencing increasing demand for the Hemolung® Respiratory Assist System (RAS) as a result of the current pandemic.
The Food and Drug Administration (FDA) granted the Company Emergency Use Authorization (EUA) designation to the Hemolung RAS for the treatment of COVID-19 patients in the second quarter of 2020. The Hemolung RAS is the only ECCO2R device currently granted an EUA for the treatment of COVID-19.
“The Hemolung RAS has enabled us to recover patients with COVID pneumonia during the pandemic. In select patients where there is a selective issue with hypercarbic respiratory acidosis while their oxygen requirements have normalized post-Veno-Venous ECMO cannulation, we have utilized Hemolung as a bridge in their recovery.
“We have noticed that these patients are able to wean off mechanical circulatory support in a gradual manner. Additionally, at a time when there was a shortage of ECMO circuits, our program has relied on utilizing this technology in stabilizing patients with severe hypercarbic respiratory acidosis while providing lung protective ventilation,” stated Dr. Bindu Akkanti, MD, Associate Professor of Medicine, Divisions of Critical Care, Pulmonary and Sleep Medicine, McGovern Medical School, and Director of Heart and Vascular Critical Care, Memorial Hermann – Texas Medical Center.
“The Hemolung RAS has given us a new tool during the current pandemic, to safely and easily treat our COVID-19 patients. We were able to rapidly introduce the Hemolung RAS to our staff and start treating patients under Emergency Use Authorization. As a smaller community hospital without an ECMO program, the ease of use of the Hemolung has played a large role in the successful deployment of ECCO2R for the treatment of COVID-19 at Palm Beach Gardens Medical Center,” stated Ribal Darwish, MD, Medical Director Critical Care Medicine, Palm Beach Gardens Medical Center.
“We are pleased to be able to assist in this fight against the COVID-19 viral disease by providing the use of the Hemolung RAS as a tool for physicians to be used in conjunction with IMV, by reducing or eliminating the potential of further lung damage caused by high ventilator driving pressures, often referred to as Ventilator Induced Lung Injury (VILI). We are treating COVID-19 patients in greater than 20 hospitals worldwide,” said Mr. Peter M. DeComo, Chairman and CEO of ALung Technologies.
In its EUA approval letter to ALung the FDA stated that it believes the Hemolung RAS has the potential to treat lung failure as an adjunct to noninvasive or invasive mechanical ventilation, to reduce hypercapnia and hypercapnic acidosis due to COVID-19, and/or to maintain normalized levels of partial pressure of carbon dioxide(PCO2) and pH in patients suffering from acute, reversible respiratory failure due to COVID-19 for whom ventilation of CO2 cannot be adequately, safely, or tolerably achieved and, in turn, may provide clinical benefit, and that there is no adequate, approved and available alternative to the emergency use of the Hemolung RAS to treat lung failure caused by COVID-19.