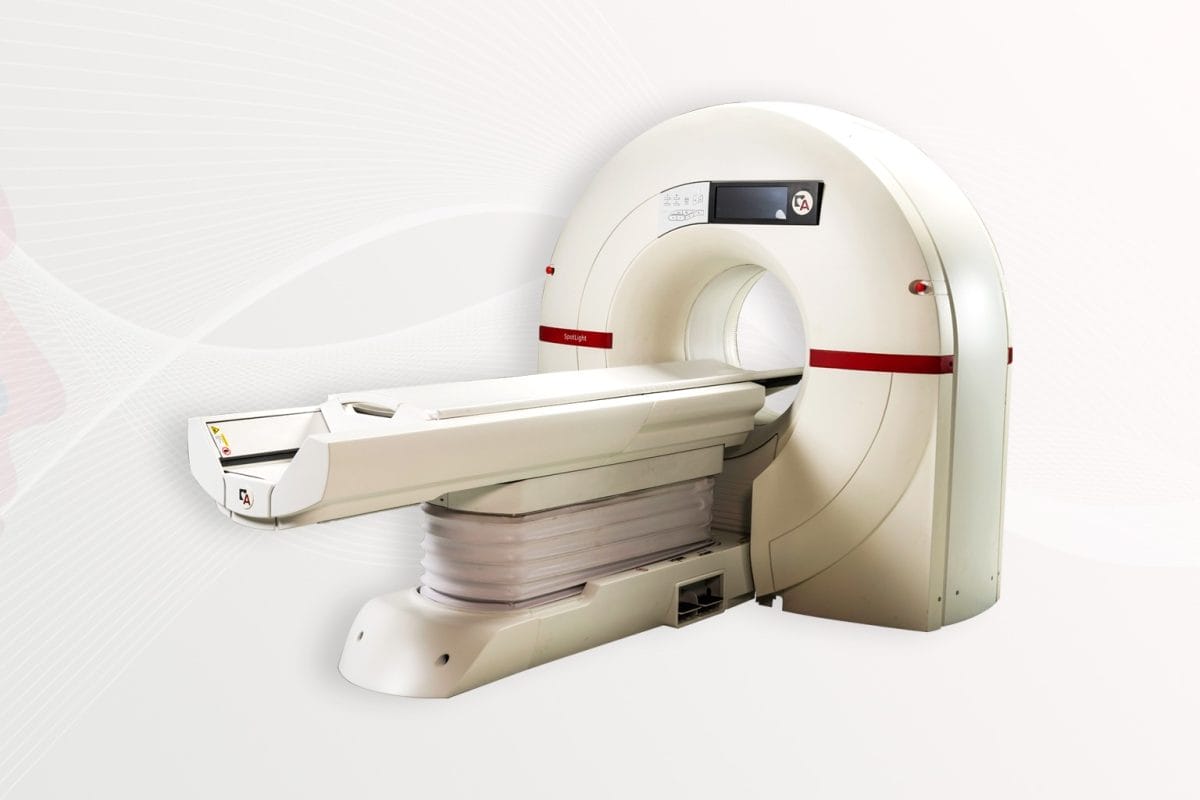
Arineta, a leader in advancing cardiovascular imaging solutions through cutting-edge technology, announces the installation of the first SpotLight™ Duo, the highest performance cardiovascular and thoracic CT scanner in the industry in a major health system in Texas. The FDA-cleared SpotLight Duo adds high resolution thoracic clinical capabilities for lung cancer and other pulmonary diseases to its cardiovascular CT technology. This noninvasive system with a reduced physical footprint allows for point-of-care access to diagnostics, therapy planning and monitoring of cardiovascular disease.
The single beat, whole heart scanner is optimized with 140 mm coverage at a rotation speed of 0.24 sec per rotation. Its deep-learning imaging reconstruction (DLIR) technology provides improved image quality by reconstructing cross-sectional images of the subject, allowing for a more complete and accurate CT scan.
“For patients in need of cardiovascular or cardiothoracic imaging, the SpotLight Duo is the first cardiothoracic CT incorporating all of Arineta’s patient centric technologies. With one heartbeat, we can provide both high-temporal as well as iso-temporal resolution images that can be used for comprehensive cardiac and thoracic CT assessment,” said Doug Ryan, CEO of Arineta. “At the same time, we are reducing radiation and contrast for scans while delivering more detailed and accurate images for better informed decision-making by physicians.”
This milestone follows Arineta’s recent installation of the first SpotLight CT, a sister product to the SpotLight Duo, at Connected Cardiovascular Care Associates in Dallas.