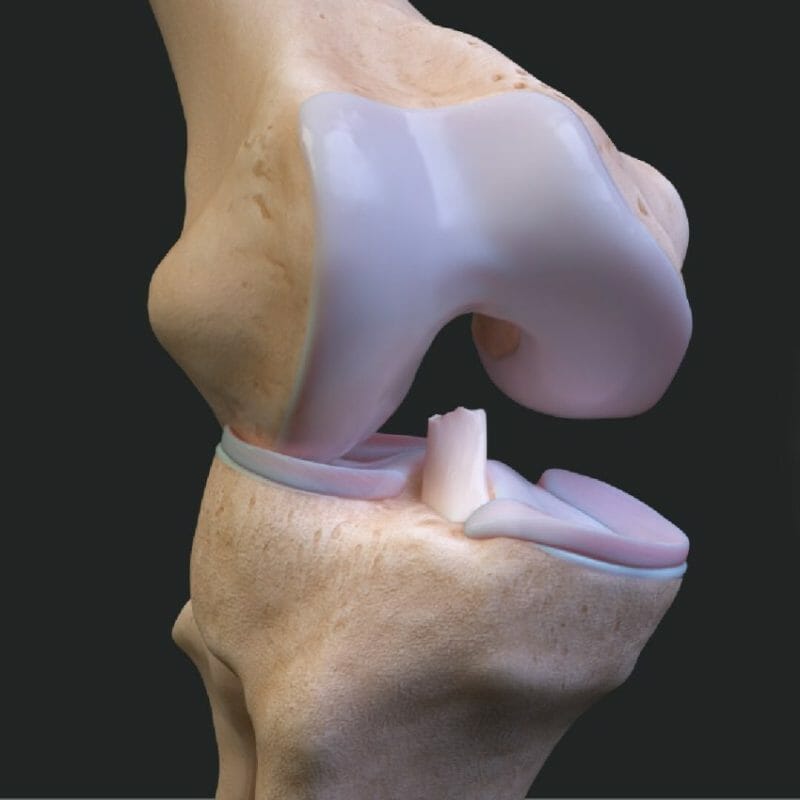
Arthrex, a global leader in minimally invasive orthopedic technology, launched a new patient-focused website, ACLTear.com, which illustrates the science of anterior cruciate ligament (ACL) injuries, highlights the benefits of knee preservation technology and provides tools to patients to connect with surgeons performing advanced, minimally invasive ACL procedures.
“As the innovation leader in arthroscopic surgery, Arthrex is defining the future for how ACL injuries are treated,” said Arthrex President and Founder Reinhold Schmieding. “ACLTear.com is a tremendous educational resource for patients who want comprehensive information based on extensive orthopedic research and real patient experiences.”
Arthrex recently released two solutions for minimally invasive ACL preservation: the ACL Repair TightRope® implant and the SwiveLock® ACL Repair Kit, the first of its kind in the orthopedic industry to gain FDA clearance. Unlike a reconstruction or complete replacement of the ACL, the new technology enabling the preservation of a patient’s own ACL may allow for an easier recovery1 with a more normal-feeling knee2 and a possible faster return to activity.3
“Arthrex is dedicated to helping patients gain a better understanding of their injuries and the techniques available to improve their outcomes,” said Senior Director of Product Management, Knee and Hip Arthroscopy Jake Jolly. “ACLTear.com is an educational hub for anyone suffering from an ACL injury to explore their treatment options and connect with expert surgeons nearby.”
Arthrex has been involved in developing ACL repair and reconstruction techniques since 1983, with the goal of creating less invasive procedures to assist patients in returning to activity more quickly. In developing these techniques, the company has thoroughly studied the procedures and associated clinical outcomes in high-impact peer-reviewed literature.1,4-6
ACL tears and sprains are among the most common sports injuries in the U.S., with an estimate of between 100,000 to 200,000 occurrences every year.7 Younger and sports-active patients are predominately affected, typically by twisting or hyperextension.
ACLTear.com offers an interactive Find a Doctor tool to help patients quickly and easily connect with surgeons in their area.