September 18, 2020
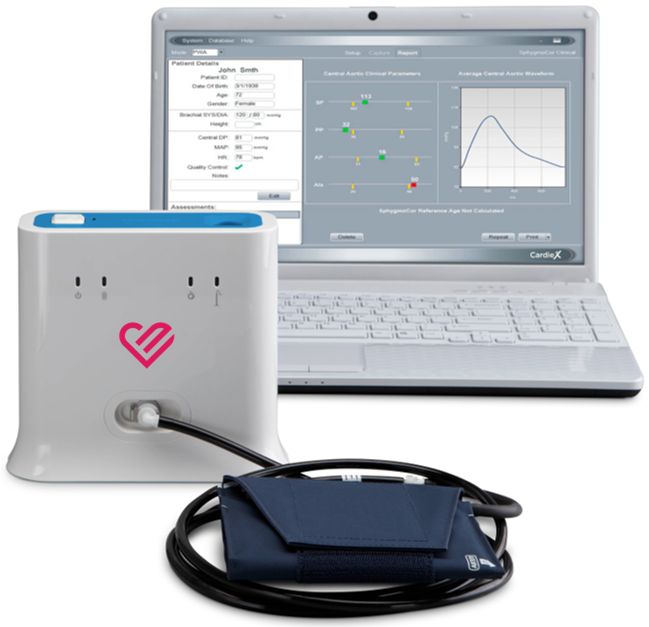
CardieX Limited announced today that CardieX subsidiary ATCOR was granted a new patent by the European Patent Office (EPO) for the Company’s proprietary SphygmoCor ® technology used in cuff-based blood pressure devices. EPO Patent Number EP2566387 further protects the company’s intellectual property in relation to the measurement of central blood pressure (BP) waveform with cardiovascular features using a brachial cuff. Patent EP2566387 specifically covers non-invasively estimating the heart’s pressure and pressure waveform related to cardiac function and arterial properties using a conventional BP cuff inflated to low pressure. The patent provides a simple tool to clinically diagnose and estimate the risk of heart disease.
Examining officers from the EPO referenced the substantial differences to existing patents when granting the new patent to ATCOR, demonstrating the unique nature of the technology. The granting of the European patent follows similar patents granted in the United States and Japan with the new European patent in force until 2034.
ATCOR’s SphygmoCor® technology is the global standard for the non-invasive measurement of arterial stiffness in medical settings and is in use at all “Top 20 USA Hospitals” and more than over 4500 installations worldwide including major pharmaceutical companies and research institutions such as The Mayo Clinic, GlaxoSmithKline, AstraZeneca, Bayer, Scripps, Johns Hopkins, and Stanford University.
ATCOR currently sells the XCEL central blood pressure device and has a partnership with SunTech Medical for the Oscar 2 ABPM device, which both incorporate ATCOR’s SphygmoCor® and brachial cuff technology. The Company also recently announced a partnership with Andon for the development of a home-based BP device with advanced cardiovascular vital signs features.