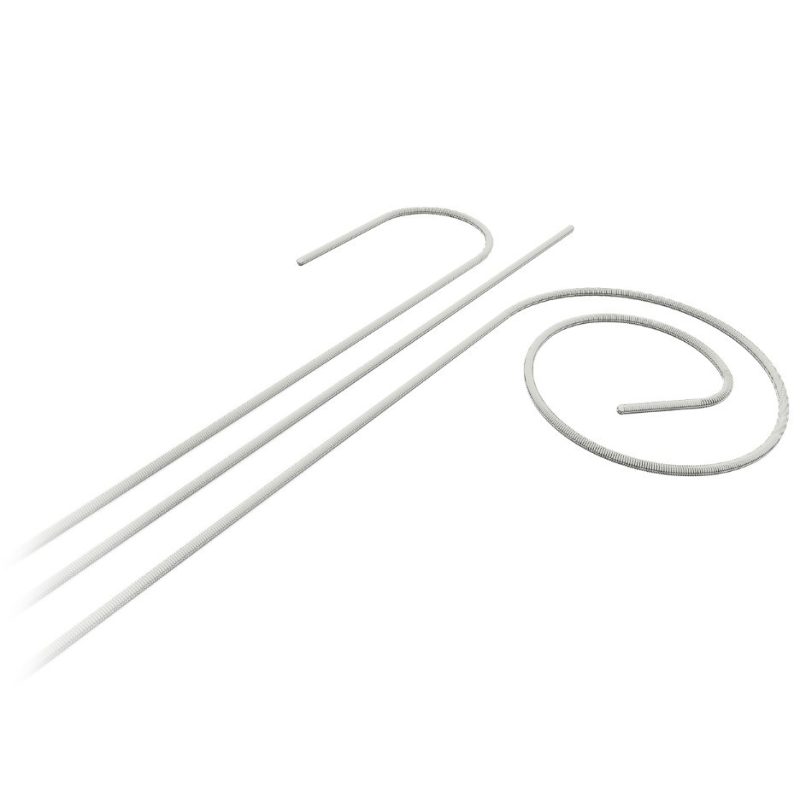
Atraverse Medical HOTWIRE™ RF guidewire device has been used for the first time in clinical practice by world-renowned cardiac electrophysiologist, Dr. Devi Nair, at St. Bernards Medical Center. The best-in-class left-heart access device received FDA 510(k) clearance earlier this year and was unveiled at the Heart Rhythm Society congress in May.
Co-founded by Dr. Steven Mickelsen, the pioneer of Farapulse pulsed field ablation technology, Atraverse’s HOTWIRE™ device is a novel radiofrequency (RF) guidewire that enables zero exchange left-heart access while acting as a rail for catheter-based therapy systems. Featuring universal sheath compatibility, the HOTWIRE™ is designed to improve patient outcomes and streamline procedural workflows.
“We are thrilled to use the HOTWIRE™ in our clinical practice and are proud to be first in the world to utilize this innovative technology,” commented Dr. Nair, who performed twelve cases after crossing the septum with the HOTWIRE™, including four atrial fibrillation ablation procedures and eight left atrial appendage closures, while utilizing five different models of transseptal sheaths. “We have been eagerly awaiting a sheath-agnostic solution like this, and we look forward to using the product in a majority of our cases going forward for left atrial access.”
Eric Sauter, COO and Co-Founder for Atraverse Medical, commented…
“Our mission is to improve efficiency and take the guesswork out of what should be simply-executed therapeutic and diagnostic procedures. Our collaboration with Dr. Nair exemplifies our dedication to taking patient care to the next level with innovative technologies.”
In addition, the company has strengthened its executive leadership team with two industry veterans in support of its commercialization plan. Jay Kelley, MBA, who joined the Atraverse Medical team as Vice President of Marketing in 2023, has been promoted to Chief Marketing Officer. Mr. Kelley has been instrumental in the development of Atraverse’s commercialization strategy to drive market adoption with a focused launch. He has more than 20 years of medical device R&D and commercialization experience, primarily focused on cardiac electrophysiology.
Most recently, in May of 2024, Brandon Allen joined Atraverse as Vice President of Sales. Mr. Allen brings two decades of experience in the cardiac electrophysiology space, serving with market leaders including Biosense and Guidant / Boston Scientific, and has propelled the company forward following the FDA 510(k) clearance of the HOTWIRE™.
“Our HOTWIRE™ device has gained the attention of several thought leaders in the field, and we are excited to have Jay and Brandon on the team leading commercialization efforts,” noted John Slump, CEO and Co-Founder of Atraverse Medical. “Their deep experience and impeccable reputations are a tremendous asset to our company.”